Oral Dantrolene Reduces Myalgia and Hyperckemia in a Child with RYR1-Related Exertional Myalgia/Rhabdomyolysis
Abstract
RYR1-related exertional myalgia/rhabdomyolysis (ERM) is an underrecognized condition, which can cause limiting muscle symptoms, and may account for more than one-third of undiagnosed rhabdomyolysis cases. Dantrolene has shown promising results in controlling muscle symptoms in individuals with ERM, however, its use in children remains poorly documented. This case report presents the successful treatment of a 5-year-old patient with ERM using oral dantrolene. The patient experienced notable improvements, including a reduction in the frequency and intensity of myalgia episodes, no hospitalizations due to rhabdomyolysis, a substantial decrease in creatine phosphokinase (CPK) levels, and enhanced performance on the 6-minute walk test. The use of dantrolene was well-tolerated, and no significant adverse effects were observed. This report adds to the existing evidence supporting the effectiveness of oral dantrolene in managing ERM, and, to the best of our knowledge, this is the first report of the use of dantrolene in a pediatric patient for controlling anesthesia-independent muscle symptoms.
INTRODUCTION
RYR1-related exertional myalgia/rhabdomyolysis (ERM) is an underdiagnosed condition, which may be responsible for more than one-third of undiagnosed rhabdomyolysis [1]. Muscle symptoms in ERM can manifest at all ages, with variable triggers, including physical activity, heat, infection, alcohol ingestion, or a combination of multiple factors [2]. There is incomplete penetrance, with a predominance of symptomatic males [2].
Pathogenic variants in skeletal muscle ryanodine receptor (RYR1) gene can cause multiple phenotypes and muscle symptoms, spanning from congenital myopathies to malignant hyperthermia susceptibility (MHS) [3]. Additionally, there’s an increasing amount of evidence that gain of function variants in RYR1, associated with MHS, frequently cause ERM, even without a MH history, suggesting that ERM may be a more common manifestation in MHS variants than clinical MH [4].
Dantrolene is a muscle relaxant used for the treatment of MH that selectively blocks the RyR1 channel, thus, relaxing the skeletal muscle. In individuals with MHS, the use of oral dantrolene has been reported to control anesthesia-independent muscle symptoms, such as awake MH and ERM since the 1980 s [5–7]. In 2023, Moreno et al. evaluated the use of oral dantrolene for myopathic symptoms in 164 MHS patients with muscle symptoms, showing that the drug was well-tolerated, with no serious adverse effects, and 87% of patients adhered to therapy and reported improvement of myalgia, fatigue, or rhabdomyolysis/hiperCKemia [8].
Pediatric use of dantrolene is recommended both for the treatment of MH and spasticity. Chronic use is recommended for children older than 5 years old, with a minimum dose of 0.5 mg/kg/dose. The main adverse reaction is hepatotoxicity, therefore frequent monitoring of Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT) is recommended [9].
This report describes the case of a 5-year-old patient diagnosed with ERM and his response to oral dantrolene, corroborating existing evidence of significant benefit with this medication.
The legal guardian of the patient provided informed consent to publish this case report.
CASE STUDY
A 2-year-old male child started presenting episodes of myalgia and gait alterations during physical activity, such as playing, running and jumping. At 3 years old, during preschool activities, he had a sudden bout of fever (38.5°C) and intense lower limb myalgia, making him unable to walk, thus prompting an emergency room (ER) visit. Lab results were suggestive of rhabdomyolysis, with a creatine phosphokinase (CPK) level of 66,413 U/L, a C-reactive protein (CRP) of 66.7 mg/L (reference value < 10), no other lab abnormality or sign of localized infection, thus being diagnosed as viral myositis.
After a few days of rest, he experienced no further episodes of fever and made a complete recovery. Additionally, his CPK levels decreased to 3,835 U/L after three days. However, the child continued to experience recurrent episodes of myalgia following a varied and unpredictable range of activities. These activities could be intense, such as playing and running, or as mild as holding a phone, engaging in small building block play, or participating in a pincer grasp activity at preschool. The mother did not associate viral illness as a trigger for the myalgia episodes since the child was generally healthy and presented no other respiratory or gastrointestinal symptoms.
The myalgia manifested in different muscle groups, including the upper limbs, lower limbs, neck, and back, depending on the triggering activity. It predominantly occurred during physical activity, although the mother also reported a few instances where the myalgia occurred a couple of days after strenuous activity. During more severe myalgia episodes, the boy typically refrained from walking, and the mother noticed an elevation in body temperature (ranging from 37-38°C), leading to visits to the emergency room. Furthermore, the boy’s CPK levels would rise significantly, ranging from 8,145 to 21,176 U/L. However, after a few days of rest, his CPK levels would typically decrease to a mean resting CPK of 2,932 U/L.
At 4 years of age, he was admitted for investigation. After an unremarkable biochemical investigation for metabolic myopathies and inflammatory diseases, the patient underwent a next-generation sequencing panel of neuromuscular diseases, which identified the likely pathogenic variant RYR1 (NM_000540.2) c.7076 G>A; p.(Arg2359Gln), in heterozygosity, previously described as associated with MHS [10, 11], and curated by ClinGen MHS Variant Curation Expert Panel, confirming the diagnosis of RYR1-related ERM.
Family history (Fig. 1A) revealed a pattern of muscle cramps among some maternal relatives. The mother (II.2) reported experiencing muscle cramps even with minor exertion, such as holding a phone or walking. Similarly, the aunt (II.3) experienced mild muscle cramps during physical activity but was able to recover after resting. Another aunt (II.4) encountered muscle cramps while playing soccer, which eventually caused her to discontinue the sport. The grandfather (I.3) experienced nightly cramps almost daily.
Fig. 1
–A: Pedigree showing segregation of the variant with the phenotype (ERM and/or muscle cramps). Individual II-3 complained of a history of mild muscle cramps, possibly a phenocopy. B: Bar chart showing CPK levels that decreased remarkably after initiating dantrolene therapy. Dantrolene was started at half of the effective dose at night to evaluate potential side effects including dizziness and fatigue. The patient tolerated it well, and the dose and time of administration were adjusted, to maintain the lowest effective dose, which was 0,5 mg/kg/day (= 10 mg of dantrolene), taken during the morning, which was the time when the patient was more active, and achieved a greater decrease in the level of CPK.
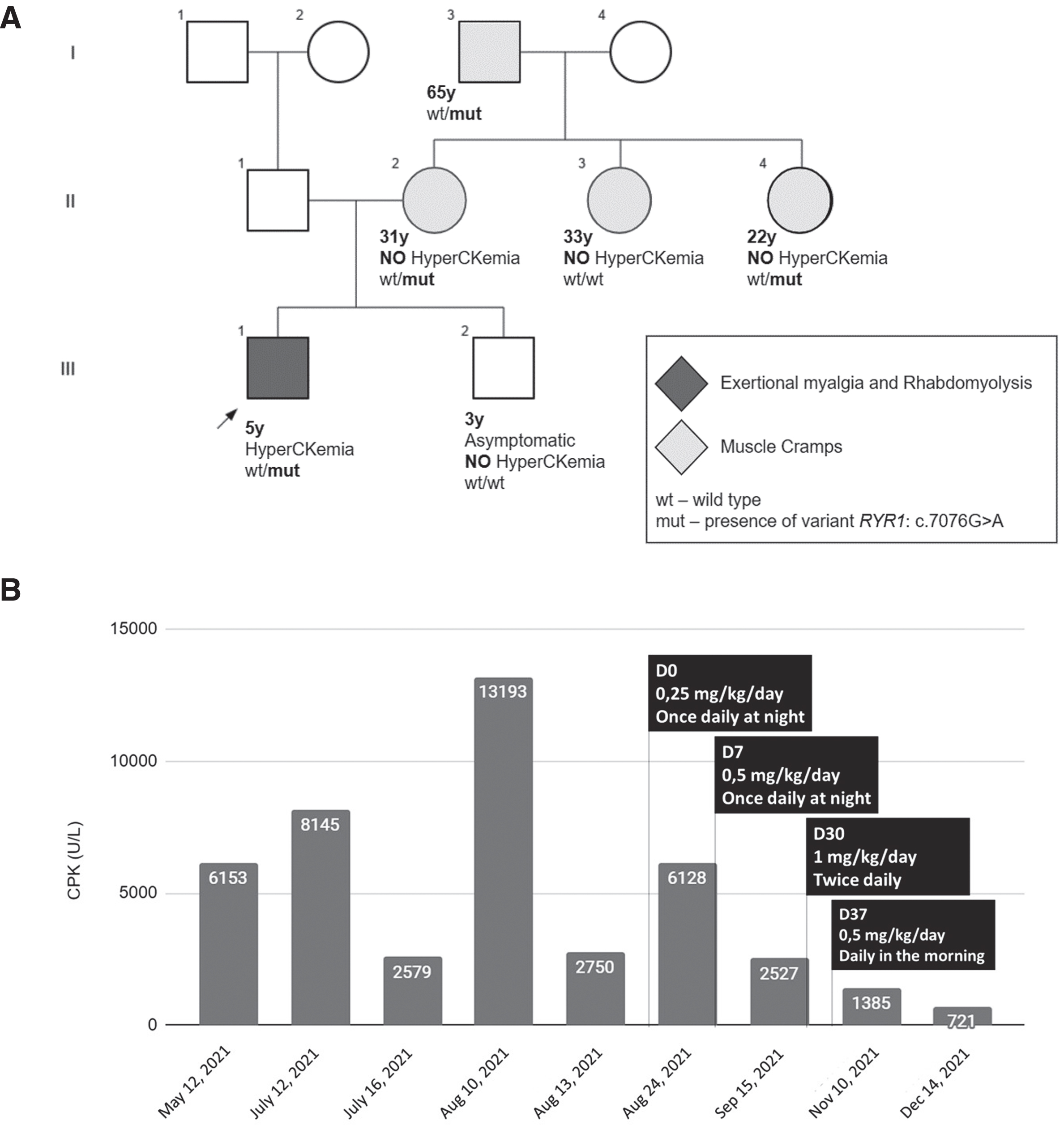
Family segregation analysis revealed that the mother, aunt II.4, and grandfather were also carriers of the variant. Notably, none of these individuals had experienced a clinical MH reaction, despite anesthesia exposure. The mother (II.2) underwent spinal anesthesia for a C-section and reported inhaling an unspecified anesthetic during a nose surgery. The grandfather (I.3) was exposed to general anesthesia for a spine surgery following an accident, but the specific anesthetics used were not documented in his medical records. Aunt II.4, on the other hand, has never been exposed to anesthesia.
Following the diagnosis of ERM, the family was advised about triggers and primary prevention of MH crisis. They did not report any other RYR1-associated symptoms, such as heat intolerance and/or bleeding abnormalities. The adult family members had learned to cope with the muscle symptoms by regulating their level of unaccustomed physical activity. However, despite their efforts to manage the child’s physical activities, he continued to experience recurrent episodes of ERM, which impaired his overall development.
As an attempt to control muscle symptoms, low-dose oral dantrolene was initiated according to the scheme detailed in Fig. 1B. Prior to dantrolene administration, the median CPK levels (interquartile range, IQR) were 6,153 U/L (8005) over a 5-month period. However, after initiating therapy, the median CPK levels decreased to 1,956 U/L (4341), indicating a significant 68% reduction. A similar trend was observed when comparing the mean CPK (standard deviation, SD) levels between the two periods. Before dantrolene administration, the mean CPK level was 6,564 U/L (4,389), whereas after therapy initiation, it decreased to 2,690 U/L (2410). The highest CPK level recorded after dantrolene administration was 6,128 U/L, which occurred following a myalgia episode triggered by running and playing on a swing two days earlier. When comparing the pre-dantrolene mean resting CPK (2932 U/L) with the post-dantrolene mean CPK, there was a reduction of 8.2%.
Moreover, the mother was advised to document the occurrence of myalgia episodes in a diary starting one month before the dantrolene trial. The analysis revealed a 50% reduction in the frequency of myalgia attacks –10 episodes in the month pre-dantrolene vs. 5 episodes in the month post-dantrolene, primarily induced by playing and walking. The family also reported a notable decrease in the intensity of myalgia attacks, enabling the child to progressively engage in activities that were previously limited by myalgia, such as extended playtime, jumping on a trampoline, and fully participating in school activities. During the period of dantrolene use, there were no hospitalization due to myalgia, whereas two hospitalizations occurred prior to initiating dantrolene treatment.
No significant adverse effects were observed throughout the treatment period. In order to monitor hepatotoxicity, AST and ALT levels were regularly measured prior to every dose adjustment, and after dose stabilization at Day 30, measurements were taken every 1 to 2 months. These evaluations revealed a progressive decrease in AST and ALT levels, corresponding to the decline in CPK levels. The mean AST level decreased from 117.5 U/L pre-dantrolene to 63.3 U/L post-dantrolene, while the mean ALT level decreased from 40.0 U/L pre-dantrolene to 25.3 U/L post-dantrolene. As expected, the administration of dantrolene in the morning, when the child was more physically active, demonstrated improved control over myalgia attacks and resulted in lower CPK levels (Fig. 1B).
Furthermore, in the 6-minute walk test (6MWT) pre-dantrolene, the distance traveled was only 295 meters, which is lower than the p3 (approximately 350 m) for 5-year-old caucasian boys [12] vs. 431 meters (p10-p25) post-dantrolene. Additionally, during the pre-dantrolene 6MWT, the child exhibited an initial Borg scale rating of 0, which increased to 0.5 by the test’s conclusion, indicating a slight perceived exertion. Conversely, in the post-dantrolene 6MWT, both the initial and final Borg scale ratings remained consistently at 0, indicating a reduced perception of exertion throughout the test.
CONCLUSION
Multiple descriptions of oral low-dose dantrolene in adults with both MHS and ERM indicate an improvement in muscle symptoms and good tolerability, without significant adverse events [5–8]. To the best of our knowledge, this is the first report of the use of dantrolene in a pediatric patient for controlling anesthesia-independent muscle symptoms. Our report corroborates previous descriptions that dantrolene is an effective medication for managing ERM. It adds further evidence about the use of dantrolene in children, in whom it was able to reduce frequency, intensity, and hospitalizations due to ERM, decreased CPK levels, and improved distance on 6MWT, which may be a useful functional parameter for therapeutic response assessment.
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interest regarding this publication.
REFERENCES
[1] | Dlamini N , Voermans NC , Lillis S , Stewart K , Kamsteeg EJ , Drost G , et al. Mutations in RYR1 are a common cause of exertional myalgia and rhabdomyolysis, Neuromuscular Disorders (2013) ;23: (7):540–8. |
[2] | Voermans NC , Snoeck M , Jungbluth H RYR1 -related rhabdomyolysis : A common but probably underdiagnosed manifestation of defective skeletal muscle ryanodine receptor dysfunction. Revue Neurologique. 2016. |
[3] | Snoeck M , van Engelen BGM , Küsters B , Lammens M , Meijer R , Molenaar JPF , et al. RYR1-related myopathies: A wide spectrum of phenotypes throughout life, Eur J Neurol (2015) ;22: (7):1094–112. |
[4] | Riazi S , Bersselaar van den LR , Islander G , Heytens L , Snoeck MMJ , Bjorksten A , et al. Pre-operative exercise and pyrexia as modifyingfactors in malignant hyperthermia (MH), Neuromuscular Disorders (2022) ;32: (8):628–34. |
[5] | Gronert GA , Thompson RL , Onofrio BM Human Malignant Hyperthermia: Awake Episodes and Correction by Dantrolene, Anesth Analg (1980) ;59: (5):377–8. |
[6] | Timmins MA , Sc B , Rosenberg H , Larach MG , Sterling C , Sc B , et al. Malignant Hyperthermia Testing in Probands without Adverse Anesthetic Reaction, Anesthesiology (2015) ;123: (3):548–56. |
[7] | Scalco RS , Voermans NC , Piercy RJ , Jungbluth H , Quinlivan R Dantrolene as a possible prophylactic treatment for RYR1- related rhabdomyolysis. Eur J Neurol. 2016;56-7. |
[8] | Ibarra Moreno CA , Kraeva N , Zvaritch E , Jungbluth H , Voermans NC , Riazi S Oral Dantrolene for Myopathic Symptoms in MalignantHyperthermia-Susceptible Patients: A 25-Year Retrospective CohortStudy of Adverse Effects and Tolerability, Anesth Analg (2023) ;136: (3):569–77. |
[9] | Lexicomp Inc Dantrolene: Drug information [Internet], 2021 [cited 2022 Jan 26]. Available from:https://www.uptodate-.2pt.-.2ptcom-.2pt/-.2ptcontents-.2pt/-.2ptdantrolene-.2pt–.2ptdrug-.2pt–.2ptinformation-.2pt?-.2ptsearch=.2ptdantrolene.2pt&source.2pt=panel.2pt_search.2pt_result.2pt&selectedTitle.2pt=.2pt1∼35&usage_type=panel.2pt&k2pt_tab.2pt=drug.2pt_general&display_rank=1 |
[10] | Klingler W , Heiderich S , Girard T , Gravino E , Heffron JJA , Johannsen S , et al. Functional and genetic characterization of clinical malignant hyperthermia crises: A multi-centre study, Orphanet Journal of Rare Diseases (2014) ;9: (8):1–15. |
[11] | Johnston JJ , Dirksen RT , Girard T , Gonsalves SG , Hopkins PM , Riazi S , et al. Variant curation expert panel recommendations for RYR1 pathogenicity classifications in malignant hyperthermia susceptibility. GENETICS in MEDICINE. 2021;1-8. |
[12] | Ulrich S , Hildenbrand FF , Treder U , Fischler M , Keusch S , Speich R , et al. Reference values for the 6-minute walk test in healthy children and adolescents in Switzerland, BMC Pulmonary Medicine (2013) ;13: (1):1. |



