Effects of Tianeptine Treatment on Depression and Cognitive Function in Patients with Alzheimer’s Disease: A 12-Month Retrospective Observational Study
Abstract
Background:
Depression is a common manifestation in Alzheimer’s disease (AD). In clinical practice, antidepressant medication is often used for depression in AD.
Objective:
We explore the effectiveness of the atypical antidepressant tianeptine compared with other conventional antidepressants in AD patients with depression in a real-life setting.
Methods:
We retrospectively identified 126 AD patients who had received antidepressant treatment for 12 months with tianeptine or other antidepressants. Subjects were divided into two groups according to the treatment they had received: tianeptine group (n = 38) or other antidepressant group (n = 88). Drug effects on depression, cognition, behavior, and functional performance were evaluated at baseline, 6, and 12 months. A Mixed Effects Model Analysis was carried out to evaluate changes in performance scores.
Results:
Both tianeptine and other antidepressants showed an antidepressant effect after 12 months with significant improvement on the Cornell Scale for Depression in Dementia, the Hamilton Depression Rating Scale, and the Neuropsychiatric Inventory-Depression subscale. A statistically significant improvement at 12 months was shown in the tianeptine group versus the other antidepressants group on most of the cognitive measures such as the Mini-Mental State Examination, the Letter and Category Fluency Test, the Rey Auditory Verbal Learning Test, and the Boston Naming Test.
Conclusion:
Our results suggest that tianeptine reduces depressive symptoms and improves cognition in AD patients. This could be considered clinically relevant and should inspire the design of future long-term randomized controlled trials that contribute to supporting the use of tianeptine for improving cognitive function in AD patients.
INTRODUCTION
Alzheimer’s disease (AD) is the major cause of dementia in the elderly, accounting from 60% to 80% of all of cases [1]. Although the cognitive and functional symptoms have been those characteristically identified in subjects with dementia, the neuropsychiatric symptoms have been taking on increasing importance in recent years. Depression represents one of the most common neuropsychiatric conditions among patients affected by AD both early in the cognitive decline and later when the dementia process is most severe [2, 3]. The prevalence of clinically significant depressive symptoms in individuals with AD, including mood changes, social withdrawal, irritability, and suicidal ideation, ranges from 10% to 86% [4], although the prevalence of depression based on accepted diagnostic criteria is estimated between 20% and 45% [5]
Depression coexistent with AD has been associated with poor quality of life [6], increased caregiver burden [7], higher use of psychoactive drugs [8], more rapid cognitive decline and limitations in activities of daily living [9, 10], increased risk of nursing-home placement [11, 12], and mortality [13].
In clinical practice, antidepressant medication is often used for the treatment of depression along with nonpharmacologic strategies in AD. However, some recent systematic reviews of antidepressants for treating depression in dementia do not provide strong support for the efficacy of antidepressants for treating depression in dementia. The evidence on remission rates favored antidepressants but was of moderate quality [14, 15]. However, despite major concerns about potential side effects and efficacy, antidepressants remain widely used in practice [16]. In fact, despite the lack of definitive evidence of their efficacy, people with AD have three times more antidepressant prescriptions than people of the same age without dementia [17]. In addition, the management of depression in these patients is complicated by comorbid medical conditions, potential drug interactions, increased vulnerability to side effects of medications, and drug costs [5, 18]. Therefore, it is of interest to investigate the effects of new treatments for depression that may in turn have benefits for the treatment of depression in AD.
In recent years, it has been shown that neurotransmitter dysfunction in depression goes beyond monoaminergic hypothesis, with glutamate becoming increasingly important [19]. Elevated glutamate levels in the central nervous system of depressed patients [20] and abnormalities of the glutamatergic receptor N-methyl-D-aspartate (NMDA) have been found in suicide victims and in patients with major depression [21]. In AD, both behavioral disturbance and cognitive impairment are thought to be associated with NMDA receptor dysfunction as increasing evidence of dysfunctional glutamatergic neurotransmission had been reported in behavioral changes and cognitive decline in such patients. Literature suggests that behavioral disturbance and cognitive impairment of AD may be associated with excitatory neurotoxic effects which result in impairment of neuronal plasticity and degenerative processes [22].
In this sense, tianeptine is an atypical antidepressant drug which has been shown to modulate glutamatergic transmission, and its effects on neuroplasticity have been extensively studied in the hippocampus and amygdala [23]. In addition, tianeptine is an efficacious μ-opioid receptor agonist and to a lesser extent, δ-opioid receptor agonist, without affecting the Kappa receptor. Tianeptine’s relationship with the μ-opioid receptor is characterized by triggering transduction mechanisms that are probably different to those of the conventional opioids, and this might be indirectly involved in the mechanism of its antidepressant action. The special action of tianeptine on opioid receptors might explain the release of dopamine in the limbic system and also participate in the modulation of glutamatergic mechanisms. Thus, the properties of tianeptine as a very unique opioid might contribute to its antidepressant properties [24].
This differential action with most of the currently in use antidepressants could explain additional properties: The glutamatergic neurons in the human brain project their axons to subcortical regions, such as the locus coeruleus, the raphe nuclei, and the substantia nigra. In those nuclei, glutamatergic neurons modulate monoaminergic pathways, allowing the glutamatergic system to participate in physiological functions such as memory and cognition and others related to neurotrophicity and neuronal plasticity [25]. Therefore, tianeptine as a glutamatergic modulator, among other mechanisms, allows us to approach depression from a different point of view than other antidepressants.
The efficacy and tolerability of tianeptine are clearly demonstrated in depressed patients [26]. In addition, several studies have shown its effects on cognition in preclinical rat models [27, 29] and clinical evidence also supports an improvement in cognitive function following treatment with tianeptine in patients diagnosed as having major depression or bipolar disorder [30, 31]. However, to date, no studies have been carried out on elderly patients with dementia.
The aim of the present study was to explore the effectiveness of tineptine compared with current antidepressant treatments on depression and cognitive functions in AD patients in a real-life setting. To this end, we carried out a retrospective analysis of service provision data over a 12-month period from the Instituto Andaluz de Neurociencia (IANEC) in a sample of people suffering from AD.
METHODS
Patient selection
We retrospectively identified patients with a diagnosis of Alzheimer’s disease (AD) attending the Alzheimer Disease Center and Memory Clinic of the Instituto Andaluz de Neurociencia (IANEC), Málaga, Spain, between January 2019 and December 2020. The diagnosis of AD was made by a neurologist or psychiatrist based on the benchmarks set forth by the National Institute on Aging Alzheimer’s Association workgroups (NIA/AA). To be included in the study all AD patients also had to have a 15-item Geriatric Depression Scale score > 5 [32]. This score is a criterion to prescribe an antidepressant at the IANEC. In this study, cases with lower scores and therefore not medicated were excluded. In addition, none of the patients included in the study had a pre-existing history of depression and this was their first depressive episode treated with antidepressants. In order to assess the possible effectiveness of tianeptine on cognitive functions in AD patients, we recruited patients clinically defined in stages 4 (mild dementia) or 5 (moderate dementia) of the Global Deterioration Scale [33] on the assumption at this level of impairment the benefits on cognition might become more evident.
The study was conducted using patients selected from the IANEC records. The data were collected by clinical investigators from the patient medical records, including treatment with antidepressants, physical examination, neurological and psychiatric examination, neuropsychological assessment, and magnetic resonance imaging (MRI). As part of the routine clinical follow-up, patients at the IANEC are evaluated for the first time upon admission and every six months including information on demographic and clinical characteristics: sex, age, educational level, AD diagnosis, personal and family history, personal psychiatric history, general medication, vascular risk factors (VRFs) such as hypertension, dyslipidemia, heart disease, diabetes, and obesity, and antidementia drugs. The duration of dementia was calculated from the initial diagnosis of AD made by a specialist to the time of diagnosis of depression at the IANEC.
Excluded from the study were patients who, in addition to the diagnosis of AD, had any evidence of focal vascular lesions (such as hematomas), strokes, normal pressure hydrocephalus; significant neurologic antecedents, such as brain trauma, brain tumors, epilepsy or inflammatory disease; those with serious systemic diseases such as hypothyroidism or chronic renal failure; those with a history of severe mental illness, personality disorder, substance abuse, or developmental disorders; absence of a complete medical history to assess the study variables, and presence of a sensory disorder (e.g., severe vision and hearing impairment), and those without a legal representative who could sign the informed consent to review their medical records.
Patients were receiving treatment for 12 months with any of the following drugs and doses: donepezil (5 or 10 mg daily), galantamine (16 or 24 mg daily), rivastigmine patch (9.5 or 13.3 mg daily), or memantine (20 mg daily). Patients also were receiving stable doses of other classes of medications including antihypertensives, anticoagulants, calcium channel blockers, diuretics, lipid-reducing agents, and antidiabetic drugs. No patient started any antipsychotic medication during the study period. Regarding antidementia drugs, no patient changed their medication and none of them took combined treatment with AChEI plus memantine during the study period.
AD subjects who received treatment for at least 12 months with any of the following drugs were considered as candidates: tianeptine (25 mg daily), escitalopram (10 to 15 mg daily), paroxetine (20 mg daily), bupropion (150 mg daily), venlafaxine (75 to 150 mg daily), and sertraline (50 mg daily). Each patient only received treatment with a single antidepressant. Subjects were divided into two groups according to the treatment they had received: Patients receiving tianeptine (n = 38) versus patients receiving any of the other antidepressant drugs above listed (n = 88). In the latter group, 22 patients (25%) were taking escitalopram, 18 patients (20%) were taking bupropion, 17 patients (19%) were taking sertraline, 16 patients (18%) were taking paroxetine, and 15 patients (17%) were taking venlafaxine. In addition to pharmacological treatment, all selected subjects participated in a psychostimulation program as part of the activities they routinely perform at the memory clinic. Briefly, the main activities are aimed at cognitive functions, personal and spatial orientation, and improvement of psychomotor skills. Patients are also encouraged to participate in recreational or occupational activities. Both groups maintained adherence to the psychostimulation program throughout the duration of the study.
During the study period, a total of 329 patients with AD were identified. From these 37 potential participants were excluded because they had insufficient documentation in their medical record, 35 had a pre-existing history of depression, 31 were taking more than one antidepressant, 25 did not maintain the same antidepressant during the 12-month follow-up, 21 initially candidates were unable to be contacted, 12 refused, and 42 were excluded according to the study criteria.
This is considered a pilot study and hence, the sample size in this retrospective study was not ascertained a priori due to the lack of appropriate information for a formal calculation. The study was approved by the local ethics committee and an informed consent was obtained from patients or their representative caregivers.
Assessment
Recorded data were collected on the effects of drugs on depression, cognition, behavior, and functional performance. Assessments were conducted at three time points: when the diagnosis of depression was made and antidepressant treatment started, and at 6 and 12 months thereafter using the following neuropsychological tests.
Depression
The Cornell Scale for Depression in Dementia (CSDD) was administered as a 19-item semi-structured interview, with scores above 10 indicating a probable major depression and scores above 18 indicating a definite major depression. It is especially designed for assessing depression in dementia patients and has internal consistency, and validity [34]. The Hamilton Depression Rating Scale (HDRS) (17 items version) must be completed at the end of a semi-structured interview by a trained clinician, and it focus basically on the somatic and behavioral components of depression. The higher the score, the higher the depression is [35]. The Neuropsychiatric Inventory (NPI) is composed of 12 subscales that evaluate the most frequent behavioral and psychological symptoms in AD patients [36]. As a secondary depression outcome measure, the depression subscale of the NPI (NPI-D) was carried out. If depression was present during the previous month, frequency, severity, and composite (frequency x severity) scores were obtained. Frequency was rated from 1 to 4 while severity could vary from 1 to 3.
Cognitive function
The Mini-Mental State Examination (MMSE) is the most commonly used test for the screening of cognitive functioning. Possible scores range from 0 to 30 points, where higher scores indicate better cognitive function [37].
The Rey Auditory Verbal Learning Test (RAVLT) is a verbal list-learning and memory test to assess verbal episodic memory. The RAVLT consists of 5 repeated learning trials of the same 15-word list, with immediate and delayed recall trials after 3 and 30 min, respectively, as well as recognition tests. In this study we used the total sum of words recalled across the five trials to measure total encoding [38].
The Symbol Digit Modalities Test (SDMT) is a widely used measure of information processing speed. The subject is presented with a page headed by a key that pairs the single digits 1–9 with nine symbols. Rows below contain only symbols, and the subject’s task is to write or orally report the correct number in the spaces below. After completing the first 10 items with guidance, the subject is timed to determine how many responses can be made in 90 s [39].
The Boston Naming Test (BNT) is the best-known neuropsychological test used widely for evaluating linguistic ability which includes object naming and word retrieval. In this study we used the abbreviated 15-item version [40].
The Trail Making Test is a tool that is used for the assessment of the ability to flexibly switch attention between competing task-set representations. The TMT comprises of two task components, TMT-A and TMT-B. The TMT-A requires the participant to draw lines and connect circled numbers in a numerical sequence. In the TMT-B, the participant is asked to draw lines to connect circled numbers and letters in an alternating numeric and alphabetic sequence. The participant is instructed to complete both task components as fast and accurately as possible without lifting the pen from the worksheet [41].
Letter fluency test (LFT) and Category fluency test (CFT) were used to assess verbal fluency. Both involve the activation of multiple cognitive processes engaging verbal knowledge and executive function to inhibit repetitions. In the LFT, subjects were instructed to say as many words as possible that begin with the letter ‘P’ for 1 min. In the CFT, the subjects were asked to list as many animals as possible within 1 min [42].
Neuropsychiatric symptoms
The study of behavioral and psychological symptoms of dementia was carried out using the NPI. A composite score for each of the 12 subscales was obtained by multiplying frequency by severity, with a maximum of 12 points. A total composite score can be obtained ranging from 0 to 144 [36].
Functional status
The patients’ performance on activities of daily life was assessed using the Interview for Deterioration in Daily Living (IDDD). Possible scores range from 33 to 99 points, where higher scores indicate worse functional ability [43].
Statistical analysis
Demographic variables were reported using the mean and standard deviation in the case of quantitative variables, and number and percentage in the case of qualitative. Baseline differences between the two treatment groups were assessed by an analysis of variance (ANOVA) or nonparametric tests, as appropriate.
A Mixed Effects Model Analysis for repeated measurements was carried out in order to be able to evaluate changes in depression, cognitive, functional and neuropsychiatric scores, and excuse participants with a missing value in some of the follow-up assessments. The effect of time (between the mean baseline measurements and each time point), treatment and treatment by time interactions were evaluated. The change scores at 6- and at 12-month follow up and the mean change scores differences within and between groups were calculated from the mixed model using estimated linear combinations.
All analyses were controlled for sex, age at baseline, and years of education. Post hoc analyses for multiple comparisons were conducted using Bonferroni’s correction. Cohen’s d standardized effect sizes were calculated and defined as small d = 0.20, medium d = 0.50, and large d = 0.80 [44]. Cohen’s d values are presented so that a positive effect size indicates improvement in the active (tianeptine) group versus control and vice versa.
SPSS Statistics (Version 25.0, IBM Corp., Armonk, NY, USA) was used for the analyses and the significance level was set at p≤0.05.
RESULTS
Demographic and baseline scores
A total of 126 patients met the inclusion criteria and their data were available for analysis (81 female, 45 male). Patients had a mean age of 75.51±5.54 years (range 65–95) and mean years of education of 6.52±1.95 (range 4–13). The education level is low because the patients in the sample belong to a generation born at a time when the educational systems in Spain were in disarray, which implies that the quality of early education was poor in this period.
All patients were Caucasian. Fifty-eight (40%) patients suffered from hypertension, 25 (18.8%) suffered from diabetes, and 44 (34.9%) suffered from dyslipidemia. Mean body mass index score was 24.2±3.2. At baseline, there were no statistically significant differences between the two groups for the demographic and clinical variables, vascular risk factors, and antidementia drugs (Table 1). Neuropsychological, neuropsychiatric, and functional performance of patients at baseline is shown in Table 2.
Table 1
Demographic and clinical characteristics of patients at baseline
| Variable | Overall (n = 126) | Tianeptine group (n = 38) | Other antidepressants group (n = 88) | p |
| Age | 75.51±5.54 | 74.87±4.44 | 75.78±5.96 | 0.342 |
| Sex | ||||
| Female | 81 (64.3) | 26 (68.4) | 55 (62.5) | 0.524 |
| Male | 45 (35.7) | 12 (31.6) | 33 (37.5) | |
| Ethnicity | ||||
| Caucasian | 126 (100) | 38 (100) | 88 (100) | |
| Marital status | ||||
| Married | 47 (37.3) | 15 (39.5) | 32 (36.4) | 0.937 |
| Single/divorced | 11 (8.7) | 3 (7.9) | 8 (9.1) | |
| Widowed | 68 (54.0) | 20 (52.6) | 48 (54.5) | |
| Duration, months | 31.97±9.58 | 29.79±6.22 | 32.91±10.61 | 0.094 |
| Education, years | 6.52±1.95 | 6.58±2.11 | 6.50±1.89 | 0.843 |
| AChEI | 79 (62.7) | 22 (57.9) | 41 (46.6) | 0.312 |
| Memantine | 47 (37.3) | 16 (42.1) | 47 (53.4) | 0.621 |
| Hypertension | 58 (40.0) | 17 (44.7) | 41 (46.6) | 0.435 |
| Dyslipidemia | 44 (34.9) | 13 (34.2) | 31 (35.2) | 0.247 |
| Diabetes | 25 (18.8) | 8 (21.1) | 17 (19.3) | 0.541 |
| Heart disease | 23 (18.2) | 11 (28.9) | 12 (13.6) | 0.371 |
| BMI, Kg/m2 | 24,2±3.2 | 25.6±2.9 | 24.8±3.0 | 0.349 |
| GDS | 8.72±2.36 | 8.86±2.41 | 8.74±2.39 | 0.463 |
Values are mean±SD or number (%). Independent samples t-test were used for continuous data and χ2 on categorical data. BMI, body mass index; GDS, Geriatric Depression Scale.
Table 2
Neuropsychological, neuropsychiatric, and functional performance of patients at baseline
| Variable | Overall (n = 126) | Tianeptine group (n = 38) | Other antidepressants group (n = 88) | p |
| MMSE | 20.88±2.00 | 21.11±1.79 | 20.78±2.09 | 0.384 |
| RAVLT | 18.34±2.88 | 18.84±2.64 | 18.13±2.97 | 0.181 |
| CFT | 8.83±1.09 | 8.87±0.70 | 8.81±1.22 | 0.723 |
| LFT | 8.67±1.06 | 8.89±0.95 | 8.57±1.09 | 0.095 |
| TMTA | 162.44±22.14 | 160.82±21.42 | 163.15±22.52 | 0.583 |
| TMTB | 245.94±31.24 | 251.32±28.52 | 243.61±32.21 | 0.185 |
| SDMT | 24.17±8.20 | 24.26±4.38 | 24.14±9.40 | 0.918 |
| BNT | 10.13±1.58 | 9.84±0.89 | 10.25±1.79 | 0.090 |
| NPI | 22.44±3.53 | 21.95±3.04 | 22.66±3.72 | 0.264 |
| NPI-D | 4.02±1.30 | 4.13±1.21 | 3.97±1.34 | 0.498 |
| HDRS | 14.87±4.12 | 14.00±4.60 | 15.24±3.86 | 0.152 |
| CSDD | 15.69±4.27 | 15.34±4.95 | 15.84±3.97 | 0.585 |
| IDDD | 52.13±13.17 | 51.71±13.61 | 52.31±13.04 | 0.820 |
Values are mean±SD or number (%); p-values from Student’s t test. MMSE, Mini-Mental State Examination; RAVLT, Rey Auditory Verbal Learning Test; CFT, Category Fluency Test; LFT, Letter Fluency Test; TMTA, Trail Making Test part A; TMTB, Trail Makin Test part B; SDMT, Symbol Digit Modalities Test; BNT, Boston Naming Test; NPI, Neuropsychiatric Inventory; NPI-D, Neuropsychiatric Inventory Depression subscale; HDRS, Hamilton Depression Rating Scale; CSDD, Cornell Scale for Depression in Dementia; IDDD, Interview for Deterioration in Daily Living Activities in Dementia.
Depression measures
Table 3 shows the results from the mixed model analysis at time 1 and time 2 for both groups. When considering the between group changes in the NPI-D, the results reveal a statistically significant time by treatment effect (F(1, 124) = 9.975, p = 0.002). The tianeptine group performed better than the other antidepressants group at 6-month follow-up (mean change score –1.63±1.07 versus –1.10±0.75, p = 0.003) and at 12-month follow-up (mean change score –1.87±1.21 versus –1.22±0.82, p = 0.002) (Fig. 1). There were no statistically significant differences between the tianeptine group and the other antidepressants group with regard to the CSDD (F(1, 124) = 0.145, p = 0.882) and the HDRS (F(1, 124) = 1.066, p = 0.582). With regard to the within group changes, both the tianeptine group and the other antidepressants group yielded significant improvement in the NPI-D, CSDD, and HDRS at 6 months (p < 0.001 in all three cases) and at 12 months (p < 0.001 in all three cases) versus baseline.
Table 3
Results from the linear mixed models for tianeptine and the other antidepressants group
| Variable | T1: 6 mo T2: 12 mo | Tianeptine group (n = 38) | Other antidepressants group (n = 88) | Between groups change score at T1 and T2 | |||||||||
| Means (SD) | Change score at T1 | Change score at T2 | p | #d | Means (SD) | Change score at T1 | Change score at T2 | p | #d | d | |||
| MMSE | T0 | 21.11 (1.79) | 0.45 (0.65) | 0.97 (0.86) | < 0.001 | 0.38 | 20.78 (2.09) | 0.09 (0.89) | 0.06 (1.13) | 0.214 | |||
| T1 | 21.55 (1.78) | 20.83 (2.19) | 0.38* | 0.35 | |||||||||
| T2 | 22.08 (1.84) | 20.80 (2.20) | 0.61** | 0.70 | |||||||||
| RAVLT | T0 | 18.84 (2.64) | 0.34 (0.58) | 0.68 (0.93) | 0.001 | 0.43 | 18.13 (2.97) | –0.07 (0.67) | –0.06 (1.01) | 0.771 | |||
| T1 | 19.18 (2.65) | 18.06 (2.89) | 0.17** | 0.27 | |||||||||
| T2 | 19.63 (2.60) | 18.10 (3.08) | 0.52** | 0.58 | |||||||||
| CFT | T0 | 8.87 (0.70) | 0.62 (0.79) | 1.26 (0.87) | < 0.0001 | 0.54 | 8.81 (1.22) | 0.23 (1.73) | 0.19 (0.67) | 0.189 | |||
| T1 | 9.42 (0.83) | 9.03 (1.21) | 0.39 | ||||||||||
| T2 | 10.16 (0.92) | 8.90 (1.18) | 0.57*** | 0.64 | |||||||||
| LFT | T0 | 8.89 (0.95) | 0.75 (0.73) | 1.40 (0.86) | < 0.0001 | 0.56 | 8.57 (1.09) | 0.18 (0.87) | 0.12 (0.84) | 0.324 | |||
| T1 | 9.58 (1.11) | 8.69 (1.32) | 0.47** | 0.58 | |||||||||
| T2 | 10.29 (1.18) | 8.62 (1.34) | 0.58*** | 0.68 | |||||||||
| TMTA | T0 | 160.82 (21.42) | –4.42 (2.80) | –8.55 (4.40) | 0.231 | 163.15 (22.52) | –0.63 (2.44) | –0.31 (23.31) | 0.311 | ||||
| T1 | 156.39 (21.34) | 162.75 (22.14) | –3.79 | ||||||||||
| T2 | 152.26 (20.90) | 163.25 (21.89) | –8.24 | ||||||||||
| TMTB | T0 | 251.32 (28.52) | –5.95 (11.05) | –3.47 (23.05) | 0.421 | 243.61 (32.21) | –2.13 (9.61) | –2.95 (24.01) | 0.351 | ||||
| T1 | 244.45 (34.69) | 242.50 (33.22) | –3.82 | ||||||||||
| T2 | 239.97 (37.58) | 240.78 (35.32) | –0.52 | ||||||||||
| SDMT | T0 | 24.26 (4.38) | –0.97 (4.20) | –1.45 (4.75) | 0.313 | 24.14 (1.00) | –1.96 (6.02) | –3.39 (7.54) | 0.244 | ||||
| T1 | 23.39 (3.65) | 23.12 (0.76) | 0.99 | ||||||||||
| T2 | 22.37 (3.06) | 20.85 (0.67) | 1.94 | ||||||||||
| BNT | T0 | 9.84 (0.14) | 0.28 (0.88) | 1.43 (0.94) | < 0.001 | 0.49 | 10.25 (0.19) | 0.15 (0.85) | 0.12 (0.97) | 0.412 | |||
| T1 | 10.13 (0.13) | 10.41 (0.21) | 0.43 | ||||||||||
| T2 | 11.21 (0.15) | 10.16 (0.21) | 0.51** | 0.57 | |||||||||
| NPI | T0 | 21.95 (3.04) | –6.74 (3.84) | –10.13 (3.81) | < 0.001 | 0.36 | 22.66 (3.72) | –3.51 (3.93) | –8.43 (3.51) | < 0.001 | 0.51 | ||
| T1 | 16.21 (3.53) | 19.48 (3.53) | –0.23 | ||||||||||
| T2 | 14.60 (3.54) | 14.22 (3.59) | 0.30 | ||||||||||
| NPI-D | T0 | 4.13 (1.21) | –1.63 (1.07) | –1.87 (1.21) | < 0.001 | 0.21 | 3.97 (1.34) | –1.10 (0.75) | –1.22 (0.82) | < 0.001 | 0.15 | ||
| T1 | 2.50 (0.73) | 2.84 (1.04) | –0.53*** | 0.57 | |||||||||
| T2 | 2.26 (0.68) | 2.73 (0.89) | –0.65*** | 0.62 | |||||||||
| HDRS | T0 | 14.00 (4.60) | –3.08 (2.36) | –4.08 (3.37) | < 0.001 | 0.34 | 15.24 (3.86) | –3.45 (3.25) | –4.86 (4.28) | < 0.001 | 0.37 | ||
| T1 | 10.89 (3.41) | 11.75 (3.49) | 0.37 | ||||||||||
| T2 | 9.87 (2.76) | 10.19 (3.14) | 0.78 | ||||||||||
| CSDD | T0 | 15.34 (0.80) | –3.58 (2.69) | –5.45 (4.15) | < 0.001 | 0.53 | 15.84 (0.42) | –3.82 (3.79) | –5.84 (4.21) | < 0.001 | 0.50 | ||
| T1 | 11.76 (0.48) | 12.04 (0.43) | 0.24 | ||||||||||
| T2 | 9.76 (0.39) | 9.84 (0.23) | 0.39 | ||||||||||
| IDDD | T0 | 51.71 (13.61) | –2.08 (14.19) | –2.34 (12.92) | 0.652 | 52.31 (13.04) | 0.15 (16.05) | 0.02 (14.61) | 0.134 | ||||
| T1 | 48.61 (17.89) | 52.26 (13.05) | –2.23 | ||||||||||
| T2 | 47.13 (14.67) | 52.68 (49.84) | –4.36 | ||||||||||
Values are means (SD); *p = 0.001, **p < 0.001, ***p < 0.0001; Adjusted for sex, age, and education; d, Cohen’s d; #Within-group effect size from T0 to T2. MMSE, Mini-Mental State Examination; RAVLT, Rey Auditory Verbal Learning Test; CFT, Category Fluency Test; LFT, Letter Fluency Test; TMTA, Trail Making Test part A; TMTB, Trail Makin Test part B; SDMT, Symbol Digit Modalities Test; BNT, Boston Naming Test; NPI, Neuropsychiatric Inventory; NPI-D, Neuropsychiatric Inventory Depression subscale; HDRS, Hamilton Depression Rating Scale; CSDD, Cornell Scale for Depression in Dementia; IDDD, Interview for Deterioration in Daily Living Activities in Dementia.
Fig. 1
Significant results from the linear mixed models for cognition and depression performances. T0 = baseline, T1 = follow-up 6 months, T2 = follow-up 12 months. MMSE, Mini-Mental State Examination; RAVLT, Rey Auditory Verbal Learning Test; CFT, Category Fluency Test; LFT, Letter Fluency Test; BNT, Boston Naming Test, NPI-D, Neuropsychiatric Inventory Depression subscale.
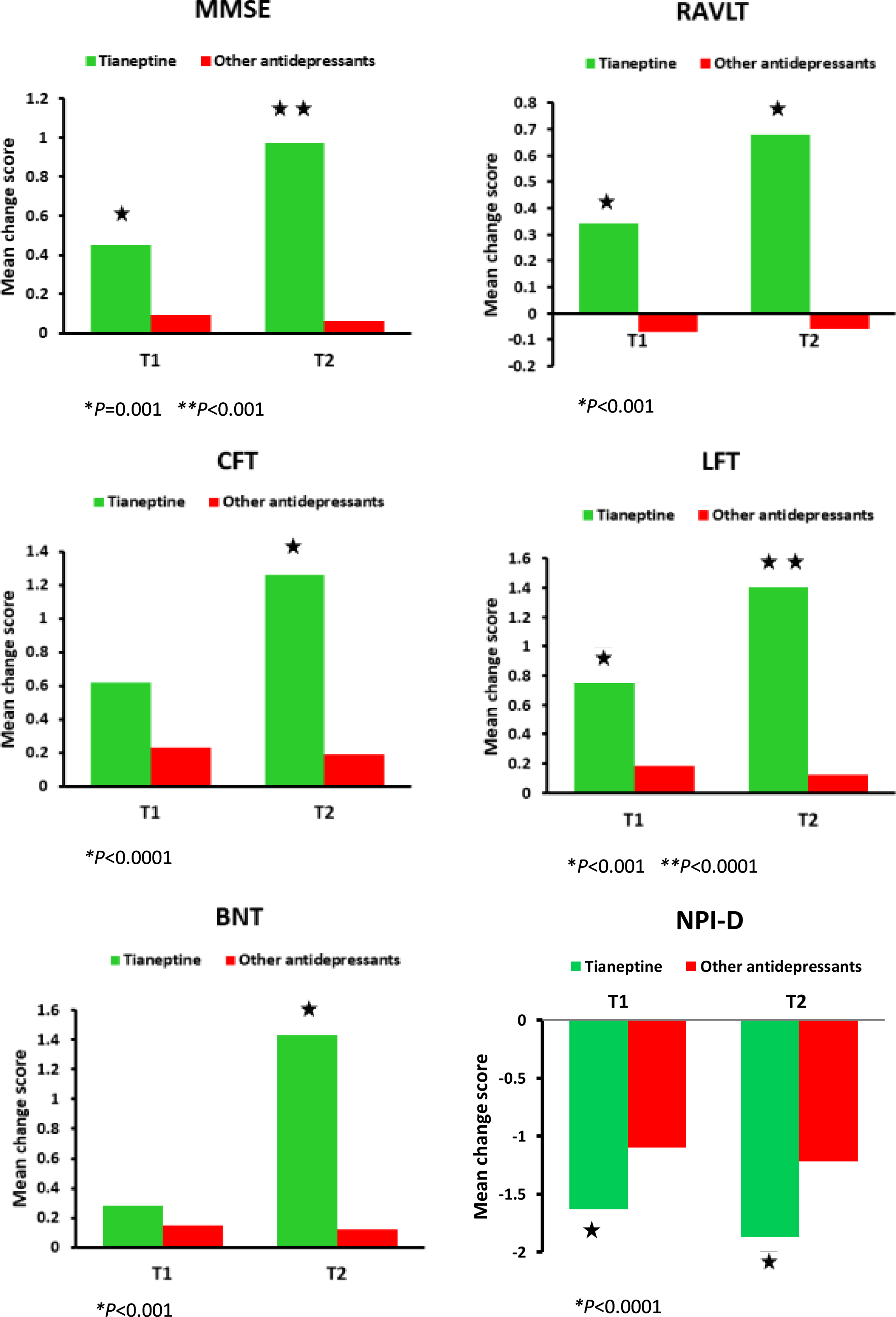
Cognitive function measures
Results from mixed model analysis at time 1 and time 2 for both groups are presented in Table 3. When considering the between group changes in the MMSE, the Mixed Model analysis showed a statistically significant time by treatment effect (F(1, 124) = 13.427, p < 0.001). The tianeptine group performed better than the other antidepressants group at 6-month follow-up (mean change score 0.57±0.68 versus 0.09±0.89, p = 0.001) and at 12-month follow-up (mean change score 0.97±0.86 versus 0.06±1.13, p < 0.001) (Fig. 1). Regarding the within group changes, the tianeptine group yielded significant improvement in the MMSE at 6 (p < 0.001) and at 12 months (p < 0.001) versus baseline. The other antidepressants group did not render significant baseline-to-endpoint differences in the MMSE.
Concerning the RAVLT, the mixed model analysis showed a statistically significant time by treatment effect (F(1, 124) = 6.390, p < 0.001). The tianeptine group scored significantly better than the other antidepressants group at 6-month follow-up (mean change score 0.34±0.58 versus –0.07±0.67, p < 0.001) and at 12-month follow-up (mean change score 0.68±0.93 versus –0.07±1.01, p < 0.001) (Fig. 1). The tianeptine group rendered significant improvement in the RAVLT at 6 (p = 0.001) and at 12 months (p = 0.001) versus baseline. The other antidepressants group did not render significant baseline-to-endpoint differences in the RAVLT.
With regard to the CFT, the mixed model analysis showed a statistically significant time by treatment effect (F(1, 124) = 12.524, p = 0.001). The tianeptine group performed better at 12 months compared to the other antidepressants group (mean change score 1.26±0.87 versus 0.39±0.47, p < 0.0001) (Fig. 1). The tianeptine group yielded significant improvement in the CFT at 12 months versus baseline (p < 0.0001). The other antidepressants group did not render significant baseline-to-endpoint differences in the CFT.
In regard to the LFT, the mixed model analysis showed a statistically significant time by treatment effect (F(1, 124) = 18.611, p < 0.0001). The tianeptine group showed better performance at 6-month follow-up (mean change score 0.85±0.73 versus 0.13±0.67, p < 0.001) and at 12-month follow-up (mean change score 1.40±0.86 versus 0.15±1.65, p < 0.0001) compared to the other antidepressants group (Fig. 1). The tianeptine group yielded significant improvement in the LFT at 6 (p < 0.0001) and 12 months (p < 0.0001) versus baseline. The other antidepressants group did not render significant baseline-to-endpoint differences in the LFT.
There was a statistically significant time by treatment effect with regard to the BNT (F(1, 124) = 12.787, p < 0.0001). The tianeptine group performed better than the other antidepressants group at 12-month follow-up (mean change score 1.43±0.94 versus –0.12±2.14, p < 0.001) (Fig. 1). The tianeptine group yielded significant improvement in the BNT at 12 months versus baseline (p < 0.001). The other antidepressants group did not render significant baseline-to-endpoint differences in the BNT.
The mixed model analysis did not show a statistically significant time by treatment effect neither for the TMTA (F(1, 124) = 1.903, p = 0.170), TMTB (F(1, 124) = 1.094, p = 0.298), or for the SDMT (F(1, 124) = 0.976, p = 0.325).
Neuropsychiatric symptoms and functional status measures
Results of the mixed model analysis at time 1 and time 2 for both groups are shown in Table 3. Concerning the NPI, the Mixed Model analysis showed a statistically significant time by treatment effect (F(1, 124) = 3.273, p < 0.001), although post hoc comparisons showed no significant differences in change scores between groups. Both the tianeptine group and the other antidepressants group yielded significant improvement in the NPI at 6 (p < 0.001 in both cases) and at 12 months versus baseline (p < 0.001 in both cases) (Table 3).
The mixed model analysis did not show a statistically significant time by treatment effect with regard to the IDDD (F(1, 124) = 0.819, p = 0.442).
Safety analysis
Tianeptine was well tolerated. The overall incidence of patients reporting adverse events throughout the study that were considered possibly related to treatment was 24.3% in the tianeptine group and 26.4% in the other antidepressants group. Table 4 shows the side effects reported in the two groups. The most commonly reported were nausea (13.16%), headache (13.16%), constipation (10.53%), and dizziness (10.53%) for tianeptine group; constipation (17.04%), nausea (14.77%), headache (13.64%), somnolence (13.64%), dizziness (11.36%), and vertigo (9.09%) in the other antidepressants group.
Table 4
Treatment adverse events reported
| Tianeptine (n = 38) | Other antidepressants (n = 88) | p Fisher’s Exact Test | |
| Nausea | 5 (13.2) | 13 (14.8) | 0.11 |
| Headache | 5 (13.2) | 12 (13.7) | 0.33 |
| Dizziness | 4 (10.5) | 10 (11.4) | 0.25 |
| Constipation | 4 (10.5) | 15 (17.0) | 0.12 |
| Diarrhea | 1 (2.6) | 4 (4.5) | 0.18 |
| Insomnia | 3 (7.9) | 4 (4.5) | 0.30 |
| Somnolence | 0 | 12 (13.6) | 0.26 |
| Fatigue | 0 | 5 (5.7) | 0.73 |
| Vertigo | 1 (2.6) | 7 (9.1) | 0.66 |
| Agitation | 2 (5.3) | 4 (4.5) | 0.17 |
| Dry mouth | 1 (2.6) | 6 (6.8) | 0.71 |
Data are number (%).
Most adverse events in all the three groups were transient, were of mild-to-moderate intensity, and resolved spontaneously. No deaths or serious adverse events occurred during the study. Clinically relevant changes over time or differences between treatment groups were not observed in clinical laboratory test results, vital signs, weight, or ECG parameters.
DISCUSSION
This retrospective study reported the treatment effects of tianeptine versus current antidepressants on depression as well as on cognitive function in patients with mild to moderate AD. To our knowledge, this is the first study evaluating the effects of tianeptine on cognitive function in this patient population.
Both treatment groups, tianeptine and other antidepressants, after 12 months showed an antidepressant effect with a decrease on CSDD, HDRS, and NPI-D total scores. However, at 12 months of follow-up a statistically significant improvement was observed in favor of tianeptine that showed greater reduction on NPI-D total scores compared to the other antidepressants group. These results are in agreement with those shown by previous open studies that have demonstrated that tianeptine has been effective in the treatment of depressive disorders in older patients and has not been shown to be inferior in efficacy to tricyclic antidepressants or SSRIs [45, 46].
Our results also indicate that after 12 months tianeptine had a beneficial effect on cognitive performance in a sample of AD patients, as shown by significant improvement compared to baseline in MMSE, RAVLT, CFT, LFT, and BNT total scores. Treatment appears to benefit performance on a variety of cognitive domains: short memory, verbal episodic memory, linguistic ability, executive functions, selective and sustained attention, and verbal knowledge. Patients in the other antidepressants group did not show a beneficial effect on cognitive function after 12 months of treatment. These results may have clinical significance in choosing drugs for depressed patients with AD.
These findings are consistent with clinical data suggesting that tianeptine, in addition to the antidepressant effect, has a procognitive effect. In an open-label, multicenter study of patients aged 65–80 years with a diagnosis of major depressive disorder and dysthymia, a response rate at three months of 76.7% was obtained and an improvement in anxiety and cognition was also observed [47]. In another open-label study on 20 subjects aged 60–69 years with mild cognitive impairment, depressive disorder, and generalized anxiety disorder, in which different cognitive aspects (gnosia, praxis, verbal ability, writing, memory, and visuospatial activity) were assessed, the treatment with a standard dose of tianeptine produced a decrease in symptoms in the three psychopathological dimensions studied (depressive, anxious, and cognitive) [48]. In a 3-month open multicenter study of 63 patients treated with tianeptine, a response rate of 76.7% was obtained in depressive symptoms. Improvements in anxiety and cognitive performance were also observed [49].
The procognitve effect of tianeptine could be explained by its neurobiological properties that involve the ability to restore normal neuroplasticity in circumscribed brain regions and to reverse impairments in synaptic glutamate transmission. The involvement of glutamate in the mechanism of action of the antidepressant tianeptine is consistent with a well-developed preclinical literature demonstrating the key role of glutamate in the mechanism of altered neuroplasticity that underlies the symptoms of depression and AD [23, 25]. The effects of tianeptine on the glutamatergic system could provide a key action in the cascade of events triggered by this unique compound and may represent the most proximal target in the pathway leading to its antidepressant efficacy [25, 50]. In addition to the above, the positive effect of tianeptine on cognition is relevant considering that the effect of antidepressants on cognitive functioning in depressed elderly patients is under debate, and that some antidepressants may have a negative effect on cognition due to their anticholinergic action.
Statistical significance on cognitive testing has been shown in the data presented in this study, but a key related issue is whether these statistically significant effects correspond to clinically significant differences in cognitive domains assessed. In this sense, the magnitude of the observed effect on cognitive dysfunction could be contextualized using standardized effect sizes. In line with this, the clinical relevance of the significant effect of tianeptine on neuropsychological test scores could be supported by the magnitude of the effect sizes with a median Cohen’s d of 0.49. However, the magnitude of the observed effect on cognitive dysfunction should be contextualized in studies in patients with AD and the present exploratory findings should be interpreted with caution and considered indicative due to the retrospective nature of the study. In addition, patients taking tianeptine showed statistically significant differences at 12 months for most cognitive performances compared to those receiving other antidepressants with a median Cohen’s d effect size of 0.63.
In line with the results shown by other studies [26, 45], the safety data collected in this study provide further support to the known good tolerability profile of tianeptine given for 12 months in a special AD patient population. No serious adverse events occurred during the study and the incidence of side effects reported was low (15.35%) the most common being nausea, somnolence, and headache. These were mild and transient and did not lead to stopping the treatment. These results were consistent with findings of previous studies. There were no changes in blood or urinary laboratory values, and no clinically meaningful changes in the physical and neurological examinations. This low frequency of side effects such as sedative, anticholinergic, and cardiovascular adverse events and short half-life time makes tianeptine particularly suitable for use in the elderly patients suffering from AD.
Despite the lack of solid scientific evidence, antidepressants are often used for depression in AD [51]. The Depression in Alzheimer’s Disease-2 trial studied 131 patients to assess the efficacy and tolerability of sertraline for depression in mild-moderate AD. Sertraline did not demonstrate efficacy for the treatment depression symptoms in patients with AD at 12 weeks of follow-up. In addition, its use was associated with an increased incidence of adverse events [52]. The study included an additional 12-week extension phase (total duration of 24 weeks) of randomized treatment for at least partial responders during the acute phase and, for ethical reasons, the option for open-label treatment for non-responders. The results showed that sertraline treatment is not associated with delayed improvement between 12 and 24 weeks [53]. In the same way the Health Technology Assessment Study of the Use of Antidepressants for Depression in Dementia trial was a large randomized controlled trial of the efficacy of sertraline and mirtazapine versus placebo in people with probable or possible AD and depression. In all three groups, an improvement in total CSDD scores was seen from week 0 to week 13, which persisted to week 39. However, sertraline and mirtazapine did not outperform placebo [54].
Some guidelines such as the Lancet International Commission on Dementia Prevention and Care recommend not starting antidepressants in people with dementia unless there is a history of depressive episodes prior to dementia or the patient has not responded to social or psychological treatment and is moderately or severely depressed [16]. A possible explanation for this lack of positive results could be related to the fact that depression in AD patients differs from depression in those without dementia in biological, psychological, and social terms [54, 55]. In this sense, depression in dementia could be situationally determined as a reaction to the impacts of dementia, be related to neurodegeneration, or be a major depressive disorder in dementia [16, 56]. Results of some studies suggest that the pathophysiology of depression in AD may be different to that of primary depression, as indicated by the lack of evidence of pathological changes in the monoaminergic nuclei in people with AD [57, 58], and may therefore explain the lack of effectiveness of conventional antidepressant treatments. An alternative hypothesis to underlying deficits in monoaminergic neurotransmitters includes deficits in glutamatergic transmission, which has led to a call for further research in identifying biomarkers and treatments for depression in AD that extend beyond the monoaminergic hypothesis [59]. In view of the above, our findings would support the positive effect of tianeptine for the management of depressive symptoms in AD, even improving other outcomes such as cognition.
In our study, all patients participated in a psychostimulation program as part of the activities they routinely perform at the memory clinic. There is evidence that symptoms of depression in patients with dementia can be improved by psychological interventions added to usual care [60, 61]. Therefore, this should be taken into account when interpreting the results of the present study.
One of the strengths of this study was the long duration of the follow-up which suggests that the clinical benefit of tianeptine may be greater after long-term use. In addition, the patients in the study underwent a comprehensive neuropsychological, behavioral, and functional evaluation with widely used outcome measures focused on reducing observation bias. In addition, our semi-annual evaluation, allowed close monitoring of clinical changes.
There are some limitations to the study that should be considered when interpreting the results. First, this was an observational and retrospective study, and the participating subjects were not randomly assigned to treatment groups. Therefore, the results obtained could be affected by this circumstance and do not allow us to conclude on causality. Second, this is a study of a single center and, consequently, the number of subjects enrolled was limited and the sample size was small, especially for tianeptine. Therefore, the results should be considered indicative. On the other hand, we analyzed the other antidepressants as a whole, without specifying any of them in particular, which would have allowed us to better quantify the extent of the improvements observed with the treatments under study. It is therefore possible that clinical differences may exist if the different antidepressants were studied individually. In addition, the cut-off point above 5 of the 15-item GDS scale has not been validated in Spain, so there is a risk that some patients with a clinical diagnosis of depression could have been excluded. Another limitation was that all subjects were Caucasian and therefore the safety and efficacy of treatments among a small subgroup of people cannot be assumed to generalize to other groups [62]. Finally, the tests selected for the study of the domains investigated are those commonly used at IANEC. Therefore, we acknowledge that they are not the only instruments available for the assessment of these domains.
Conclusions
Tianeptine showed benefits on cognition and depressive symptoms in patients suffering from AD. These positive effects probably provide additional benefits by targeting different pathophysiological mechanisms compared to other antidepressants. In this study tianeptine presented very promising results in AD patients with mood disorders and provides evidence that many patients will benefit from antidepressants that include, though not exclusively, a glutamate-based mode of action. This study should inspire the design of future long-term randomized controlled trials that contribute to supporting the use of tianeptine for improving cognitive function in AD patients. In addition, this study could aid clinicians in the treatment of AD, benefit the patients concerned and provide reliable references for broad application.
DISCLOSURE STATEMENT
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/21-5630r3).
REFERENCES
[1] | Alzheimer’s Association ((2015) ) Alzheimer’s disease facts and figures. Alzheimers Dement 11: , 332–384. |
[2] | Chemerinski E , Petracca G , Sabe L , Kremer J , Starkstein SE ((2001) ) The specificity of depressive symptoms in patients with Alzheimer’s disease. Am J Psychiatry 158: , 68–72. |
[3] | Lyketsos CG , Del Campo L , Steinberg M , Miles Q , Steele CD , Munro C , Rabins PV ((2003) ) Treating depression in Alzheimer disease. Arch Gen Psychiatry 60: , 737–746. |
[4] | Zubenko GS , Zubenko WN , McPherson S , Spoor E , Marin DB , Farlow MR , Sunderland T ((2003) ) A collaborative study of the emergence and clinical features of the major depressive syndrome of Alzheimer’s disease. Am J Psychiatry 160: , 857–866. |
[5] | Lyketsos CG , Lopez O , Jones B , Fitzpatrick AL , Breitner J , DeKosky S ((2002) ) Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: Results from the cardiovascular health study. JAMA 288: , 1475–1483. |
[6] | Tatsumi H , Nakaaki S , Torii K , Shinagawa Y , Watanabe N , Murata Y , Sato J , Mimura M , Furukawa TA ((2009) ) Neuropsychiatric symptoms predict change in quality of life of Alzheimer disease patients: A two-year follow-up study. Psychiatry Clin Neurosci 63: , 374–384. |
[7] | De Ronchi D , Bellini F , Berardi D , Serretti A , Ferrari B , Dalmonte E ((2005) ) Cognitive status, depressive symptoms, and health status as predictors of functional disability among elderly persons with low-to-moderate education: The Faenza Community Aging Study. Am J Geriatr Psychiatry 13: , 672–685. |
[8] | Murman DL , Chen Q , Powell MC , Kuo SB , Bradley CJ , Colenda CC ((2002) ) The incremental direct costs associated with behavioral symptoms in AD. Neurology 59: , 1721–1729. |
[9] | García-Alberca JM , Lara JP , Berthier ML , Cruz B , Barbancho MA , Green C , González-Barón S ((2011) ) Can impairment in memory, language and executive functions predict neuropsychiatric symptoms in Alzheimer’s disease (AD)? Findings from a cross-sectional study. Arch Gerontol Geriatr 52: , 264–269. |
[10] | Holtzer R , Scarmeas N , Wegesin DJ , Albert M , Brandt J , Dubois B , Hadjigeorgiou GM , Stern Y ((2005) ) Depressive symptoms in Alzheimer’s disease: Natural course and temporal relation to function and cognitive status. J Am Geriatr Soc 53: , 2083–2089. |
[11] | Dorenlot P , Harboun M , Bige V , Henrard JC , Ankri J ((2005) ) Major depression as a risk factor for early institutionalization of dementia patients living in the community. Int J Geriatr Psychiatry 5: , 471–478. |
[12] | Kales HC , Chen P , Blow FC , Welsh DE , Mellow AM ((2005) ) Rates of clinical depression diagnosis, functional impairment, and nursing home placement in coexisting dementia and depression. Am J Geriatr Psychiatry 13: , 441–449. |
[13] | Mehta KM , Yaffe K , Langa KM , Sands L , Whooley MA , Covinsky KE ((2003) ) Additive effects of cognitive function and depressive symptoms on mortality in elderly community living adults. J Gerontol A Biol Sci Med Sci 58: , M461–M467. |
[14] | Dudas R , Malouf R , McCleery J , Dening T ((2018) ) Antidepressants for treating depression in dementia. Cochrane Database Syst Rev 8: , CD003944. |
[15] | Orgeta V , Tabet N , Nilforooshan R , Howard R ((2017) ) Efficacy of antidepressants for depression in Alzheimer’s disease: Systematic review and meta-analysis. J Alzheimers Dis 58: , 725–733. |
[16] | Livingston G , Sommerlad A , Orgeta V , Costafreda SG , Huntley J , Ames D , Ballard C , Banerjee S , Burns A , Cohen-Mansfield J , Cooper C , Fox N , Gitlin LN , Howard R , Kales HC , Larson EB , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbæk G , Teri L , Mukadam N ((2017) ) Dementia prevention, intervention, and care. Lancet 390: , 2673–2734. |
[17] | Laitinen ML , Lonnroos E , Bell JS , Lavikainen P , Sulkava R , Hartikainen S ((2015) ) Use of antidepressants among community-dwelling persons with Alzheimer’s disease: A nationwide register-based study. Int Psychogeriatr 27: , 669–672. |
[18] | Herrmann N ((2001) ) Recommendations for the management of behavioral and psychological symptoms of dementia. Can J Neurol Sci 28: (Suppl 1), S96–S107. |
[19] | Duman RS , Aghajanian GK , Sanacora G , Krystal JH ((2016) ) Synaptic plasticity and depression: New insights from stress and rapid acting antidepressants. Nat Med 22: , 238–249. |
[20] | Levine J , Panchalingam K , Rapoport A , Gershon S , McClure RJ , Pettegrew JW ((2000) ) Increased cerebrospinal fluid glutamine levels in depressed patients. Biol Psychiatry 47: , 586–593. |
[21] | Law AJ , Deakin JF ((2001) ) Asymmetrical reductions of hippocampal NMDAR1 glutamate receptor mRNA in the psychoses. Neuroreport 12: , 2971–2974. |
[22] | Huang YJ , Lin CH , Lane HY , Tsaid GE ((2012) ) NMDA Neurotransmission dysfunction in behavioral and psychological symptoms of Alzheimer’s disease. Curr Neuropharmacol 10: , 272–285. |
[23] | Alamo C , Garcia P , Lopez F , Zaragozá C ((2019) ) Tianeptine, an atypical pharmacological approach to depression. Rev Psiquiatr Salud Ment (Engl Ed) 12: , 170–186. |
[24] | Gassaway MM , M-L Rives ML , Kruegel AC , Javitch JA , D Sames D ((2014) ) The atypical antidepressant and neurorestorative agent tianeptine is a μ-opioid receptor agonist. Transl Psychiatry 4: , e411. |
[25] | McEwen BS , Chattarji S , Diamond DM , Jay TM , Reagan LP , Svenningsson P , Fuchs E ((2010) ) The neurobiological properties of Tianeptine (Stablon): From monoamine hypothesis to glutamatergic modulation. Mol Psychiatry 15: , 237–249. |
[26] | Guelfi JD , Dulcire C , Le Moine P , Tafani A ((1992) ) Clinical safety and efficacy of tianeptine in 1,858 depressed patients treated in general practice. Neuropsychobiology 25: , 140–148. |
[27] | Reagan LP , Hendry RM , Reznikov LR , Piroli GG , Wood GE , McEwen BS , Grillo CA ((2007) ) Tianeptine increases brain-derived neurotrophic factor expression in the rat amygdala. Eur J Pharmacol 565: , 68–75. |
[28] | Conrad CD , Galea LA , Kuroda Y , McEwen BS ((1996) ) Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci 110: , 1321–1334. |
[29] | McEwen BS , Olié JP ((2005) ) Neurobiology of mood, anxiety, and emotions as revealed by studies of a unique antidepressant: Tianeptine. Mol Psychiatry 10: , 525–537. |
[30] | Nickel T , Sonntag A , Schill J , Zobel AW , Ackl N , Brunnauer A , Murck H , Ising M , Yassouridis A , Steiger A , Zihl J , Holsboer F ((2003) ) Clinical and neurobiological effects of tianeptine and paroxetine in major depression. J Clin Psychopharmacol 23: , 155–168. |
[31] | Jeon HJ , Woo JM , Lee SH , Kim EJ , Chung S , Ha JH , Fava M , Mischoulon D , Kim JH , Heo JY , Yu BH ((2014) ) Improvement in subjective and objective neurocognitive functions in patients with major depressive disorder: A 12-week, multicenter, randomized trial of tianeptine versus escitalopram, the CAMPION study. J Clin Psychopharmacol 34: , 218–225. |
[32] | Sheikh JI , Yesavage JA ((1986) ) Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin Gerontol 5: , 165–173. |
[33] | Reisberg B , Ferris SH , De Leon MD , Crook T ((1982) ) The global deterioration scale for assessment of primary degenerative dementia. Am J Psychiatry 139: , 1136–1139. |
[34] | Yesavage JA , Brink TL , Rose TL , Lum O , Huang V , Adey M , Leirer VO ((1982) ) Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res 17: , 37–49. |
[35] | Hamilton MJ ((1960) ) A rating scale for depression. Neurol Neurosurg Psychiatry 23: , 56–62. |
[36] | Cummings JL ((1997) ) The Neuropsychiatric Inventory: Assessing psychopathology in dementia patients. Neurology 48: (Suppl. 6), S10–S16. |
[37] | Folstein MF , Folstein SE , McHugh PR ((1975) ) Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[38] | Rey A ((1958) ) L’Examen Clinique in Psychologie, Presses Universitaires de France, Paris. |
[39] | Smith A ((1982) ) Symbol digit modalities test: Manual,Western Psychological Services, Los Angeles. |
[40] | Kaplan E , Goodglass H , Weintraub S ((2000) ) The Boston Naming Test, 2nd ed, Lippincott Williams & Wilkins, Philadelphia. |
[41] | Reitan RM , Wolfson D ((1983) ) The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation 2nd ed, Neuropsychology Press, Tucson. |
[42] | Peña-Casanova J ((2005) ) Test Barcelona Revisado, Masson, Barcelona. |
[43] | Teunisse S , Derix MM ((1991) ) Measurement of activities of daily living in patients with dementia living at home: Development of a questionnaire. Tijdschr Gerontol Geriatr 22: , 53–59. |
[44] | Cohen J ((1988) ) Statistical Power Analysis for the Behavioral Sciences. Routledge Academic, New York, NY. |
[45] | Vuković O , Marić NP , Britvić D , Cvetić T , Damjanović A , Prostran M , Jašović-Gašić M ((2009) ) Efficacy, tolerability and safety of tianeptine in special populations of depressive patients. Psychiatr Danub 21: , 194–198. |
[46] | Kasper S , Olié JP ((2002) ) A meta-analysis of randomized controlled trials of tianeptine versus SSRI in the short-term treatment of depression. Eur Psychiatry 17: , 331–340. |
[47] | Chapuy P , Cuny G , Delomier Y , Galley P , Michel JP , Pareaud M , Marey C ((1991) ) Depression in elderly patients. Value of tianeptine in 140 patients treated for 1 year. Presse Med 20: , 1844–1852. |
[48] | Karpukhin IB ((2009) ) Use of Coaxil (tianeptine) in elderly patients with combined mild cognitive and depressive-anxiety disorders. Neurosci Behav Physiol 39: , 53–56. |
[49] | Saiz-Ruiz J , Montes JM , Álvarez E , Cervera S , Giner J , Guerrero J , Seva A , Dourdil F , López-Ibor JJ ((1998) ) Tianeptine therapy for depression in the elderly. Prog Neuropsychopharmacol Biol Psychiatry 22: , 319–329. |
[50] | Kasper S , McEwen BS ((2008) ) Neurobiological and clinical effects of the antidepressant tianeptine. CNS Drugs 22: , 15–26. |
[51] | Nelson JC , Devanand DP ((2011) ) A systematic review and meta-analysis of placebo-controlled antidepressant studies in people with depression and dementia. J Am Geriatr Soc 59: , 577–585. |
[52] | Rosenberg PB , Drye LT , Martin BK , Frangakis C , Mintzer JE , Weintraub D , Porsteinsson AP , Schneider LS , Rabins PV , Munro CA , Meinert CL , Lyketsos CG ; DIADS-2 Research Group ((2010) ) Sertraline for the treatment of depression in Alzheimer disease. Am J Geriatr Psychiatry 18: , 136–145. |
[53] | Weintraub D , Rosenberg PB , Drye LT , Martin BK , Frangakis C , Mintzer JE , Porsteinsson AP , Schneider LS , Rabins PV , Munro CA , Meinert CL , Lyketsos CG ; DIADS-2 Research Group ((2010) ) Sertraline for the treatment of depression in Alzheimer’s disease: Week-24 outcomes. Am J Geriatr Psychiatry 18: , 332–340. |
[54] | Banerjee S , Hellier J , Dewey M , Romeo R , Ballard C , Baldwin R , Bentham P , Fox C , Holmes C , Katona C , Knapp M , Lawton C , Lindesay J , Livingston G , McCrae N , Moniz-Cook E , Murray J , Nurock S , Orrell M , O’Brien J , Poppe M , Thomas A , Walwyn R , Wilson K , Burns A ((2011) ) Sertraline or mirtazapine for depression in dementia (HTA-SADD): A randomised, multicentre, doubleblind, placebo-controlled trial. Lancet 378: , 403–411. |
[55] | Zubenko GS , Moossy J ((1988) ) Major depression in primary dementia. Clinical and neuropathologic correlates. Arch Neurol 45: , 1182–1186. |
[56] | Livingston G , Huntley J , Sommerlad A , Ames D , Ballard C , Banerjee S , Brayne C , Burns A , Cohen-Mansfield J , Cooper C , Costafreda SG , Dias A , Fox N , Gitlin LN , Howard R , Kales HC , Kivimäki M , Larson EB , Ogunniyi A , Orgeta V , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbæk G , Teri L , Mukadam N ((2020) ) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396: , 413–446. |
[57] | Hendricksen M , Thomas AJ , Ferrier IN , Ince P , O’Brien JT ((2004) ) Neuropathological study of the dorsal raphe nuclei in late-life depression and Alzheimer’s disease with and without depression. Am J Psychiatry 161: , 1096–1102. |
[58] | Thomas AJ , Hendriksen M , Piggott M , Ferrier IN , Perry E , Ince P , O’Brien JT ((2006) ) A study of the serotonin transporter in the prefrontal cortex in late-life depression and Alzheimer’s disease with and without depression. Neuropathol Appl Neurobiol 32: , 296–303. |
[59] | Khundakar AA , Thomas AJ ((2015) ) Neuropathology of depression in Alzheimer’s disease: Current knowledge and the potential for new treatments. J Alzheimers Dis 44: , 27–41. |
[60] | Orgeta V , Qazi A , Spector AE , Orrell M ((2014) ) Psychological treatments for depression and anxiety in dementia and mild cognitive impairment. Cochrane Database Syst Rev 2014: , CD009125. |
[61] | Orgeta V , Qazi A , Spector A , Orrell M ((2015) ) Psychological treatments for depression and anxiety in dementia and mild cognitive impairment: Systematic review and meta-analysis. Br J Psychiatry 207: , 293–298. |
[62] | Manly JJ , Glymour MM ((2021) ) What the aducanumab approval reveals about Alzheimer disease research. JAMA Neurol 78: , 1305–1306. |



