Increased Surgical Complications in Smokers Undergoing Radical Cystectomy1
Abstract
Background:
Not only is smoking a risk factor for the development of bladder cancer, it has also been implicated in increasing surgical morbidity and mortality. In general, the demographic and clinical characteristics of smokers are different to non-smokers which can bias the results of the impact of smoking.
Objective:
To evaluate the impact of smoking on radical cystectomy outcomes.
Methods:
Radical cystectomy cases were identified in the National Surgical Quality Improvement Program database from 2007–2015. Smokers were matched with non-smokers using propensity scores in a 1:1 ratio. Multivariate logistic regression was performed to evaluate the overall incidence of Clavien III-V complications. Secondary analysis was performed for the incidence of each complication recorded in NSQIP.
Results:
A total of 850 smokers undergoing radical cystectomy were matched to 850 non-smokers. The matching process improved the balance of covariates between smokers and non-smokers. The overall incidence of Clavien III-V complications was higher in smokers (13.1% vs 7.4%, p < 0.001). This corresponded to an adjusted odds ratio of 1.9 [95% CI 1.4–2.6, p = 0.028]. Other comorbid conditions worsened post-operative complications amongst smokers. When evaluating each complication recorded in the database, smokers had a higher incidence of wound dehiscence, pneumonia and myocardial infarction.
Conclusion:
Current smokers have a greater risk of morbidity following radical cystectomy. This should be considered when evaluating safety of surgery and patients should be counselled accordingly. Furthermore, even a short period of pre-operative smoking cessation can improve surgical outcomes.
INTRODUCTION
Bladder cancer is the ninth most commonly diagnosed cancer worldwide with incidence trends mirroring those of smoking prevalence [1]. It is estimated that smokers have greater than a two-fold increased risk of developing bladder cancer than non-smokers and population attributable risk for ever smoking is approximately 50% [2]. Not only is smoking associated with increased bladder cancer carcinogenesis, biological aggressiveness is also greater amongst smokers. Heavy smokers have a higher burden of muscle-invasive disease than non-smokers OR of 7.2 (95% CI, 4.5–11.6) [3]. The gold-standard management option for muscle-invasive disease is radical cystectomy (RC) and bilateral lymphadenectomy. However, this is relatively morbid procedure with an estimated complication rate of 31.3%, including a 2.7% risk of death [4].
Inhalation of tobacco smoke has a multitude of adverse effects on the human body. Although the underlying mechanisms are not completely understood, there is established association between smoking and atherogenesis, which leads to cardiovascular disease. Inflammatory processes that been implicated in potentiating cardiovascular pathology [5] are also involved in the development of obstructive pulmonary diseases [6]. Furthermore, the chemicals inhaled in cigarette smoke impairs the immune system, and subsequently, healing following stress or injury [7]. Given these pathophysiological effects, a smoking history is a recognised risk factor of peri-operative morbidity and mortality across surgical procedures [RR 1.5, 95% CI 1.3–1.7] [8]. However, amongst patients undergoing RC, the association between smoking and peri-operative outcomes is not clearly defined [4, 9, 10]. Smoking status has been shown to not affect the likelihood of surgical complications following RC in multivariable logistical regression analysis [4, 10]. This relationship may be not be clearly defined in bladder cancer patients partly due to the high prevalence of smokers in this population. Additionally, there are a number of pitfalls with using regression methods to evaluate the effect of a variable especially when multiple confounders are being adjusted for simultaneously and the number of events is low, as is the case with severe surgical complications [11]. Furthermore, there may be inherent differences in patient characteristics between smokers and non-smokers, which would influence the estimate of effect. These shortcomings can be addressed by using propensity scores that can balance observed covariates between smokers and non-smokers and improve the robustness of the calculated effect.
This study aims to characterize the effect of current smoking as an independent risk factor regardless of other coexisting comorbidities on radical cystectomy outcomes by performing a propensity-matched analysis.
METHODS
Study population
The American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database from 2007–2015 was used in this study. Highly trained clinical chart abstractors at over 600 participating institutions review individual medical charts and record pre-operative information and 30-day peri-operative outcomes. Information in this database has been demonstrated to be highly reliable [12].
Radical cystectomy patients were identified in the database using Current Procedural Terminology (CPT) codes. Only cases for which the indication was bladder cancer, identified through International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes, were included for analysis. Furthermore, only elective procedures and localized cases were included. Cases with missing information on smoking status were also excluded. This study was exempt from review by an Institutional Review Board.
Outcomes
The primary outcome was the incidence of 30-day Clavien III-V complications which was calculated using previous definitions [13]. Complications of any grade in addition to wound, respiratory, cardiac, central nervous system and genitourinary complications were all evaluated as secondary endpoints. Similar to the classification system used by Borad and colleagues, wound complications included superficial surgical site infection (SSI), deep incisional SSI and/or organ/space SSI [14]. Respiratory events were defined as pneumonia, unplanned intubation, pulmonary embolism and ventilator requirement greater than 48 hours. Cardiac arrest and myocardial infarction were classed as cardiac complications.
Statistical analysis
Differences in clinical characteristics between smokers and ex-/non-smokers were compared using Student’s t test for continuous variables and chi-square test for categorical variables. Propensity score analysis with direct matching was then performed to address the difference in baseline characteristics between the groups. The propensity score was developed by including all pre-operative patient characteristics. Smokers were then matched to non-smokers using the nearest neighbour method in a 1:1 ratio without replacement. Balance of covariates was assessed both numerically and graphically using the standardized difference of means of the propensity score and a histogram of score distribution, respectively [15]. The unadjusted and adjusted incidence of the outcomes of interest were then compared between smokers and non-smokers. All p values were two-sided and statistical significance set at the 0.05 level. Data analysis was performed in R (R Foundation for Statistical Computing, Vienna, Austria) version 3.4 using the ‘MatchIt’ package [16].
RESULTS
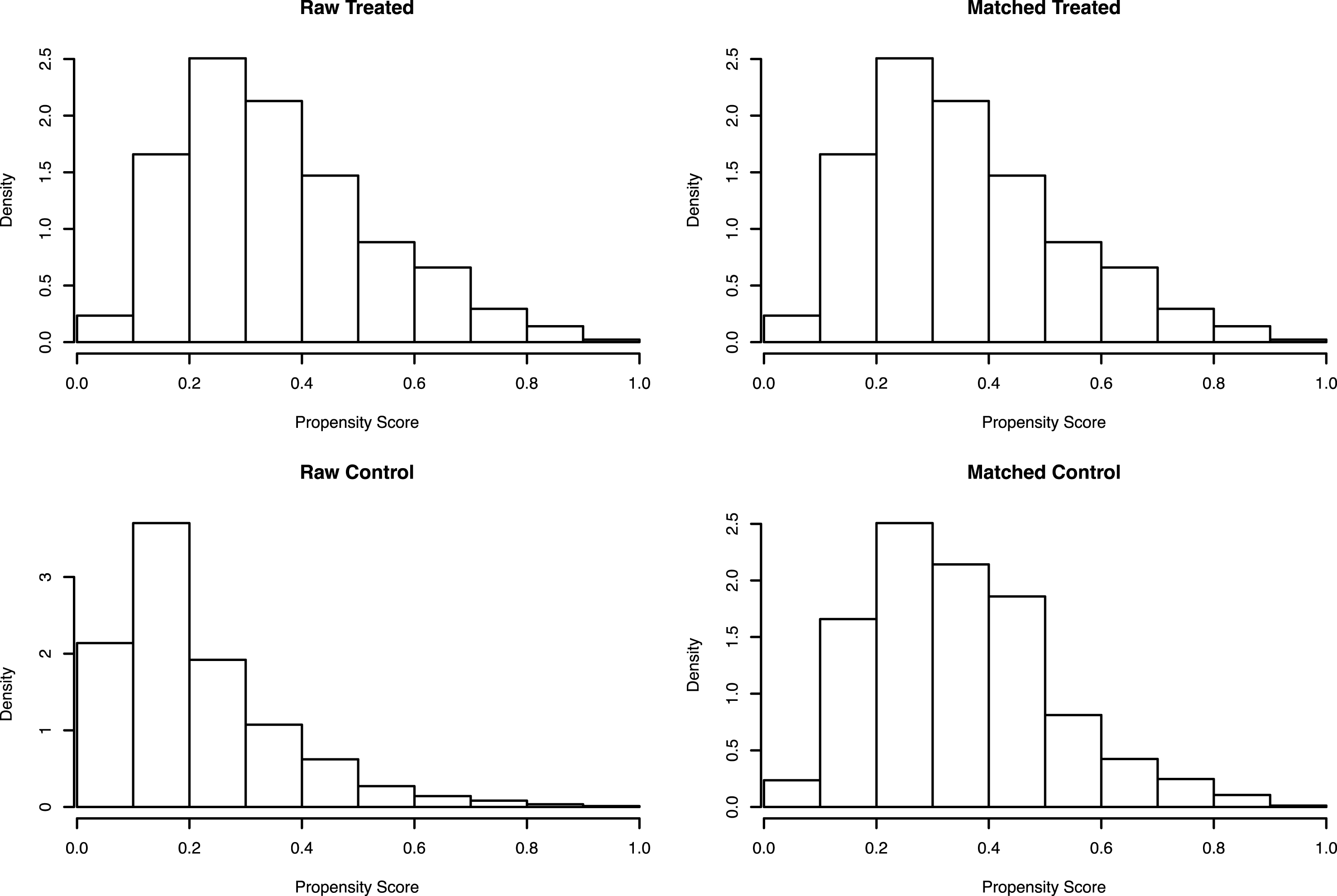
Based on the inclusion criteria outlined above, 2,541 complete cases of radical cystectomy for bladder cancer were identified. Of this cohort, 33.4% (n = 850) were classified as current smokers. Patient demographics prior to propensity score matching is outlined in Table 1. Smokers were matched with ex-/non-smokers in 1:1 ratio, thus resulting in 1,691 unmatched controls. Demographic characteristics after matching are outlined in Table 2 and the distribution of propensity scores between smokers and non-smokers are depicted in Fig. 1.
Table 1
Patient demographics prior to propensity score matching
| All patients, n (%) | Smokers, n (%) | Non-Smokers, n (%) | p value | |
| Median age (IQR) | 69 (62–76) | 64 (57–70) | 71 (64–77) | <0.001 |
| Gender, n (%) | 0.455 | |||
| Male | 4,008 (82.2) | 971 (81.5) | 3,037 (82.4) | |
| Female | 869 (17.8) | 221 (18.5) | 648 (17.6) | |
| Race, n (%) | 0.233 | |||
| White | 4,189 (94.2) | 1,029 (93.5) | 3,160 (94.4) | |
| Minority | 258 (5.8) | 72 (6.5) | 186 (5.6) | |
| Mean BMI, kg/m2 (SE) | 28.5 (0.1) | 27.3 (0.2) | 29.0 (0.1) | <0.001 |
| Comorbidities, n (%) | ||||
| Diabetes | 996 (20.4) | 188 (15.8) | 808 (21.9) | <0.001 |
| Hypertension | 2,989 (61.3) | 618 (51.9) | 2,371 (64.3) | <0.001 |
| Steroid use | 160 (3.3) | 31 (2.6) | 129 (3.5) | 0.121 |
| Weight loss | 123 (2.5) | 42 (3.5) | 81 (2.2) | 0.014 |
| Bleeding disorder | 183 (3.8) | 41 (3.4) | 142 (3.9) | 0.512 |
| Chronic heart failure | 33 (0.7) | 8 (0.7) | 25 (0.7) | 0.979 |
| Renal failure | 11 (0.2) | 2 (0.2) | 9 (0.2) | 0.618 |
| Functionally dependent | 71 (1.5) | 18 (1.5) | 53 (1.4) | 0.856 |
| ASA category, n (%) | 0.986 | |||
| 1–2 | 1,241 (25.5) | 305 (25.6) | 936 (25.4) | |
| 3 | 3,375 (69.3) | 823 (69.1) | 2,552 (69.4) | |
| 4 | 255 (5.2) | 63 (5.3) | 192 (5.2) | |
| Type of diversion, n (%) | <0.001 | |||
| Non-continent | 3,824 (80.1) | 878 (75.8) | 2,946 (81.5) | |
| Continent | 949 (19.9) | 280 (24.2) | 669 (18.5) |
Table 2
Patient demographics after to propensity score matching
| All patients, n (%) | Smokers, n (%) | Non-Smokers, n (%) | p value | |
| Median age (IQR) | 63 (57–70) | 63 (56–70) | 64 (57–70) | 0.782 |
| Gender, n (%) | 0.284 | |||
| Male | 1,393 (81.9) | 688 (80.9) | 705 (82.9) | |
| Female | 307 (18.1) | 162 (19.1) | 145 (17.1) | |
| Race, n (%) | 1.00 | |||
| White | 1,586 (93.3) | 793 (93.3) | 793 (93.3) | |
| Minority | 114 (6.7) | 57 (6.7) | 57 (6.7) | |
| Mean BMI, kg/m2 (SE) | 27.7 (0.1) | 27.9 (0.2) | 27.6 (0.2) | 0.222 |
| Comorbidities, n (%) | ||||
| Diabetes | 255 (15.0) | 130 (15.3) | 125 (14.7) | 0.734 |
| Hypertension | 900 (52.9) | 447 (52.6) | 453 (53.3) | 0.771 |
| Steroid use | 57 (3.4) | 21 (2.5) | 36 (4.2) | 0.042 |
| Weight loss | 60 (3.5) | 60 (3.5) | 30 (3.5) | 1.00 |
| Bleeding disorder | 66 (3.9) | 35 (4.1) | 31 (3.7) | 0.615 |
| Chronic heart failure | 12 (0.7) | 6 (0.7) | 6 (0.7) | 1.00 |
| Renal failure | 6 (0.4) | 2 (0.2) | 4 (0.5) | 0.409 |
| Functionally dependent | 27 (1.6) | 14 (1.7) | 13 (1.5) | 0.846 |
| ASA category, n (%) | 0.992 | |||
| 1–2 | 439 (25.8) | 220 (25.9) | 219 (25.8) | |
| 3 | 1,166 (68.6) | 582 (68.5) | 584 (68.7) | |
| 4 | 95 (5.6) | 48 (5.7) | 47 (5.5) | |
| Type of diversion, n (%) | 0.409 | |||
| Non-continent | 1,251 (73.6) | 633 (74.5) | 618 (72.7) | |
| Continent | 449 (26.4) | 217 (25.5) | 232 (27.3) |
Fig. 1
Distribution of propensity scores before and after matching.
The overall incidence of Clavien III-V complications in the matched cohort was 10.2% (n = 174). The unadjusted incidence was greater amongst smokers than non-smokers (13.1% vs 7.4%, p < 0.001). This translates to a relative risk of 1.8 [95% CI 1.3–2.4]. After adjusting for clinical characteristics, smoking status was still a significant predictor of a Clavien III-V complications [OR 1.9, 95% CI 1.4–2.6, p = 0.028]. The interplay between smoking and other covariates on complications are outlined in Table 3. Relative to non-smokers, smokers without the comorbidity of interest had a higher incidence of complications; and those smokers with the comorbidity experienced an even higher complication rate. Similarly, smokers from a minority group had a higher incidence of Clavien III-V complications than smokers of Caucasian descent or non-smokers.
Table 3
Relationship between smoking status and comorbidities on Clavien III-V complications
| Clavien III-V | p | |
| Non-smokers [ref] | 7.40% | |
| Smoker &non-diabetic | 12.80% | <0.001 |
| Smoker &diabetic | 14.60% | |
| Smoker &non-minority status | 13.00% | <0.001 |
| Smoker &minority status | 14.00% | |
| Smoker &functionally independent | 12.90% | <0.001 |
| Smoker &functionally dependent | 21.43% | |
| Smoker &no chronic heart failure | 12.80% | <0.001 |
| Smoker &chronic heart failure | 50.00% | |
| Smoker &no hypertension | 9.20% | <0.001 |
| Smoker &hypertension | 16.55% | |
| Smoker &no renal failure | 12.97% | <0.001 |
| Smoker &renal failure | 50.00% | |
| Smoker &no steroid use | 12.91% | <0.001 |
| Smoker &steroid use | 19.05% | |
| Smokers &no weight loss | 13.05% | <0.001 |
| Smoker &weight loss | 13.33% | |
| Smoker &no bleeding disorder | 12.82% | <0.001 |
| Smoker &bleeding disorder | 19.35% |
Results for the secondary endpoint evaluated are outlined in Table 4. Smoking status was significantly associated with wound dehiscence, overall wound complications, myocardial infarction and major bleeding events.
Table 4
Secondary endpoints results
| Overall Incidence | Smokers | Non-smokers | p value | Smoking adjusted odds ratio [95% CI] | |
| Wound complications | 253 (14.9) | 144 (16.9) | 109 (12.8) | 0.017* | 1.4 [1.1–1.9]* |
| Superficial SSI | 96 (5.7) | 53 (6.2) | 43 (5.1) | 0.293 | 1.3 [0.8–1.9] |
| Deep incisional SSI | 42 (2.5) | 26 (3.1) | 16 (1.9) | 0.117 | 1.7 [0.6–3.1] |
| Organ/space SSI | 92 (5.4) | 51 (6.0) | 41 (4.8) | 0.283 | 1.3 [0.8–1.9] |
| Wound dehiscence | 56 (3.3) | 36 (4.2) | 20 (2.4) | 0.028* | 1.9 [1.1–3.3]* |
| Pulmonary Complications | 124 (7.3) | 67 (7.9) | 57 (6.7) | 0.351 | 1.2 [0.8–1.8] |
| Pneumonia | 54 (3.2) | 33 (3.9) | 21 (2.5) | 0.095 | 1.6 [0.9–2.8] |
| Unplanned intubation | 47 (2.8) | 27 (3.2) | 20 (2.4) | 0.299 | 1.4 [0.8–2.5] |
| Pulmonary embolism | 37 (2.2) | 13 (1.5) | 24 (2.8) | 0.065 | 0.7 [0.4–1.2] |
| Prolonged ventilator time | 29 (1.7) | 16 (1.9) | 13 (1.5) | 0.574 | 1.2 [0.6–2.6] |
| Cardiovascular complications | 88 (5.2) | 46 (5.4) | 42 (4.9) | 0.661 | 1.1 [0.7–1.7] |
| Stroke/CVA | 7 (0.4) | 2 (0.2) | 5 (0.6) | 0.248 | 0.4 [0.1–2.1] |
| Cardiac arrest requiring CPR | 15 (0.9) | 10 (1.2) | 5 (0.6) | 0.191 | 2.4 [0.8–7.5] |
| Myocardial infarction | 20 (1.2) | 17 (2.0) | 3 (0.4) | <0.001* | 6.1 [1.8–21.3]* |
| DVT/thrombophlebitis | 52 (3.1) | 21 (2.5) | 31 (3.7) | 0.158 | 0.7 [0.4–1.2] |
| Other | |||||
| Acute renal failure | 22 (1.3) | 14 (1.7) | 8 (0.9) | 0.195 | 1.9 [0.4–8.3] |
| Progressive renal insufficiency | 33 (1.9) | 15 (1.8) | 18 (2.1) | 0.598 | 0.8 [0.4–1.6] |
| Urinary tract infection | 160 (9.4) | 75 (8.8) | 85 (10.0) | 0.406 | 0.9 [0.6–1.2] |
| Bleed requiring transfusion | 615 (36.2) | 283 (33.3) | 332 (39.1) | 0.013* | 0.8 [0.6–0.9]* |
| Sepsis | 163 (9.6) | 83 (9.8) | 80 (9.4) | 0.805 | 1.1 [0.8–1.5] |
| Septic shock | 39 (2.3) | 20 (2.4) | 19 (2.2) | 0.871 | 1.1 [0.6–2.0] |
[i] *Denotes statistical significance, p < 0.05.
DISCUSSION
This study provides a comprehensive overview of the risk profile of current smoking on radical cystectomy outcomes. Compared to non- and ex-smokers, individuals that are currently smoking have nearly a two-fold increase in risk of experiencing a major complication following radical cystectomy. Although this relationship has not been previously clearly characterized in the population of bladder cancer patients undergoing radical cystectomy, it is consistent with the broader surgical literature where smokers were reported to have an increased risk of peri-operative morbidity and mortality. Musallam and colleagues demonstrated that amongst individuals undergoing major surgery, smokers have an increased risk of mortality [OR 1.17, 95% CI 1.10–1.24], arterial events (myocardial infarction and stroke) [OR 1.65, 95% CI 1.51–1.81] and respiratory complications [OR 1.45, 95% CI 1.40–1.51] [17]. A dose-response relationship was observed by Hawn and colleagues where the incidence of pulmonary complications was highest amongst individuals with greater than a 20-year pack-year history [18]. This differs from other studies evaluating perioperative outcomes after cystectomy in which smoking status did not act as a significant predictor of complications [4, 9, 10]. In contrast to previous studies, which were not primarily designed to quantify the effect of smoking and utilized regression analyses to estimate the effect, the present study provides more robust estimates by using a propensity score method which balances observed covariates between smokers and non-smokers. The difference in baseline clinical characteristics between smokers and non-smokers (Table 1) suggests that confounding factors are contributing the lack of difference observed in previous studies and supports the need for a matched analysis. Furthermore, this study observed that additional comorbid conditions amongst smokers amplify the risk of experiencing a complication and thus emphasizes the importance of optimizing management of chronic diseases prior to surgery, especially in those that smoke. Likewise, smokers from minority groups displayed a higher incidence of Clavien III-V complications than smokers from Caucasian descent. A multitude of factors may contribute to this disparity including differences in the biological response to tobacco smoke [19–21], smoking patterns [22, 23] and/or access to high-quality healthcare [24, 25]. The findings in the current study can be employed to aid clinical decision-making when determining risk of radical cystectomy and also assists in counselling patients of this risk.
The data outlining the increase in peri-operative morbidity and mortality associated with smoking can be harnessed by clinicians to motivate individuals to quit smoking, if they have not already done so following cancer diagnosis. While smoking status should not preclude, or even delay surgery, there is high-quality evidence that suggest that even a short-period of cessation could improve outcomes. In a randomized controlled trial, smokers that were assigned to the cessation group consisting of counselling and nicotine replacement therapy that commenced four weeks prior to surgery and continued for four weeks after, had a 49% [95% CI 3–73] relative risk reduction in post-operative complications compared to controls who continued smoking [26]. The number needed to treat in the aforementioned trial was 5 [95% CI 3–40] thus highlighting the considerable effect of changing smoking habits pre-operatively. Another randomized trial by Møller et al. reported a relative risk of 0.34 [95% CI 0.17–0.58] for any complication in smokers that were in the cessation group compared to controls [27]. The largest effect observed in that cohort was for wound related complications where there was an 83% risk reduction in those that abstained from smoking [RR 0.16, 95% CI 0.05–0.52]. The results of the current study support the observed higher risk of complications amongst current smokers. It should be noted that trials which have examined smoking cessation intervention that were implemented within four weeks prior surgery were not as effective in preventing complications and thus suggests that cessation interventions need to commence at least one month before surgery to maximise benefit [28]. From a practical aspect it would be prudent to encourage smoking cessation earlier in the clinical timeline, such as at the time of haematuria workup or cancer diagnosis which especially provides an opportunity to implement lifestyle changes [29–31], so that the harms of smoking can be halted and the benefits of cessation can be fully realized.
There are certain limitations that should be considered when interpreting the results of this study. This study only focuses on cases which underwent radical cystectomy for bladder cancer and thus may not be generalizable to other procedures. An attempt was made to distinguish ex-smokers and non-smokers but this was not feasible due to pack-year data being missing in 84.2% (n = 4,108) of included cases and thus it is likely that the true difference between smokers and non-smokers is greater than observed in this study. Additionally, participation in NSQIP is voluntary and subsequently the patient population and/or outcomes may not reflect those seen in non-NSQIP institutions. Furthermore, propensity score matching can only balance covariates recorded in the database and thus it is possible that other unmeasured confounders could be impacting outcomes.
CONCLUSION
Current smokers face nearly a two-fold increase in surgical complications, particularly wound dehiscence and myocardial infarction, following radical cystectomy. As a result, smoking cessation should be encouraged during bladder cancer work-up to optimize surgical outcomes.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
ACKNOWLEDGMENTS
NJS has received support from the Cloverfields Foundation and The Institute for Prostate and Urologic Cancers (University of Minnesota).
REFERENCES
[1] | Antoni S , Ferlay J , Soerjomataram I , Znaor A , Jemal A , Bray F. . Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. European Urology. (2017) ;71: (1):96–108. |
[2] | Freedman ND , Silverman DT , Hollenbeck AR , Schatzkin A , Abnet CC. . Association between smoking and risk of bladder cancer among men and women. Jama. (2011) ;306: (7):737–45. |
[3] | Jiang X , Castelao JE , Yuan J-M , et al. Cigarette Smoking and Subtypes of Bladder Cancer. International Journal of Cancer Journal International du Cancer. (2012) ;130: (4):896–901. |
[4] | Gandaglia G , Varda B , Sood A , et al. Short-term perioperative outcomes of patients treated with radical cystectomy for bladder cancer included in the National Surgical Quality Improvement Program (NSQIP) database. Canadian Uro-logical Association Journal. (2014) ;8: (9-10):E681–E687. |
[5] | Yanbaeva DG , Dentener MA , Creutzberg EC , Wesseling G , Wouters EFM . Systemic Effects of Smoking. Chest. (2007) ;131: (5):1557–66. |
[6] | Yoshida T , Tuder RM . Pathobiology of Cigarette Smoke-Induced Chronic Obstructive Pulmonary Disease. Physiological Reviews. (2007) ;87: (3):1047–82. |
[7] | Sopori M . Effects of cigarette smoke on the immune system. Nature Reviews Immunology. (2002) ;2: :372. |
[8] | Gronkjaer M , Eliasen M , Skov-Ettrup LS , et al. Preoperative smoking status and postoperative complications: A systematic review and meta-analysis. Annals of Surgery. (2014) ;259: (1):52–71. |
[9] | Lavallee LT , Schramm D , Witiuk K , et al. Peri-Operative Morbidity Associated with Radical Cystectomy in a Multicenter Database of Community and Academic Hospitals. PLoS ONE. (2014) ;9: (10):e111281. |
[10] | Sood A , Kachroo N , Abdollah F , et al. An Evaluation of the Timing of Surgical Complications Following Radical Cystectomy: Data From the American College of Surgeons National Surgical Quality Improvement Program. Urology. (2017) ;103: (Supplement C):91–8. |
[11] | Cepeda MS , Boston R , Farrar JT , Strom BL . Comparison of Logistic Regression versus Propensity Score When the Number of Events Is Low and There Are Multiple Confounders. American Journal of Epidemiology. (2003) ;158: (3):280–7. |
[12] | Shiloach M , Frencher SK Jr , Steeger JE , et al. Toward robust information: Data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. Journal of the American College of Surgeons. (2010) ;210: (1):6–16. |
[13] | Chimukangara M , Frelich MJ , Bosler ME , Rein LE , Szabo A , Gould JC . The Impact of Frailty on Outcomes of Paraesophageal Hernia Repair. The Journal of Surgical Research. (2016) ;202: (2):259–66. |
[14] | Borad NP , Merchant AM . The effect of smoking on surgical outcomes in ventral hernia repair: A propensity score matched analysis of the National Surgical Quality Improvement Program data. Hernia: The Journal of Hernias and Abdominal Wall Surgery. (2017) ;21: (6):855–67. |
[15] | Stuart EA . Matching methods for causal inference: A review and a look forward. Statistical science: A Review Journal of the Institute of Mathematical Statistics. (2010) ;25: (1):1–21. |
[16] | Ho D , Imai K , King G , Stuart E , Whitworth A . Package ‘MatchIt’. In:(2017) . |
[17] | Musallam KM , Rosendaal FR , Zaatari G , et al. Smoking and the risk of mortality and vascular and respiratory events in patients undergoing major surgery. JAMA Surgery. (2013) ;148: (8):755–62. |
[18] | Hawn MT , Houston TK , Campagna EJ , et al. The attributable risk of smoking on surgical complications. Annals of Surgery. (2011) ;254: (6):914–20. |
[19] | Fagan P , Moolchan ET , Pokhrel P , et al. Biomarkers of Tobacco Smoke Exposure in Racial/Ethnic Groups at High Risk for Lung Cancer. American Journal of Public Health. (2015) ;105: (6):1237–45. |
[20] | Rubinstein ML , Shiffman S , Rait MA , Benowitz NL. . Race, gender, and nicotine metabolism in adolescent smokers. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco. (2013) ;15: (7):1311–5. |
[21] | Perez-Stable EJ , Herrera B , Jacob P 3rd , Benowitz NL . Nicotine metabolism and intake in black and white smokers. Jama. (1998) ;280: (2):152–6. |
[22] | Cox LS , Okuyemi K , Choi WS , Ahluwalia JS . A Review of Tobacco Use Treatments in U.S. Ethnic Minority Populations. American Journal of Health Promotion: AJHP. (2011) ;25: (5 0):S11–S30. |
[23] | Delva J , Tellez M , Finlayson TL , et al. Cigarette Smoking Among Low-Income African Americans: A Serious Public Health Problem. American Journal of Preventive Medicine. (2005) ;29: (3):218–20. |
[24] | Breslin TM , Morris AM , Gu N , et al. Hospital factors and racial disparities in mortality after surgery for breast and colon cancer. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. (2009) ;27: (24):3945–50. |
[25] | Rangrass G , Ghaferi AA , Dimick JB . Explaining racial disparities in outcomes after cardiac surgery: The role of hospital quality. JAMA Surgery. (2014) ;149: (3):223–7. |
[26] | Lindstrom D , Sadr Azodi O , Wladis A , et al. Effects of a perioperative smoking cessation intervention on postoperative complications: A randomized trial. Annals of Surgery. (2008) ;248: (5):739–45. |
[27] | Møsller AM , Villebro N , Pedersen T , Tønnesen H . Effect of preoperative smoking intervention on postoperative complications: A randomised clinical trial. The Lancet. (2002) ;359: (9301):114–7. |
[28] | Thomsen T , Tonnesen H , Okholm M , et al. Brief smoking cessation intervention in relation to breast cancer surgery: A randomized controlled trial. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco. (2010) ;12: (11):1118–24. |
[29] | Demark-Wahnefried W , Aziz NM , Rowland JH , Pinto BM . Riding the crest of the teachable moment: Promoting long-term health after the diagnosis of cancer. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. (2005) ;23: (24):5814–30. |
[30] | Lauridsen SV , Thomsen T , Kaldan G , Lydom LN , Tonnesen H . Smoking and alcohol cessation intervention in relation to radical cystectomy: A qualitative study of cancer patients’ experiences. BMC Cancer. (2017) ;17: (1):793. |
[31] | Westmaas JL , Newton CC , Stevens VL , Flanders WD , Gapstur SM , Jacobs EJ . Does a Recent Cancer Diagnosis Predict Smoking Cessation? An Analysis From a Large Prospective US Cohort. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. (2015) ;33: (15):1647–52. |



